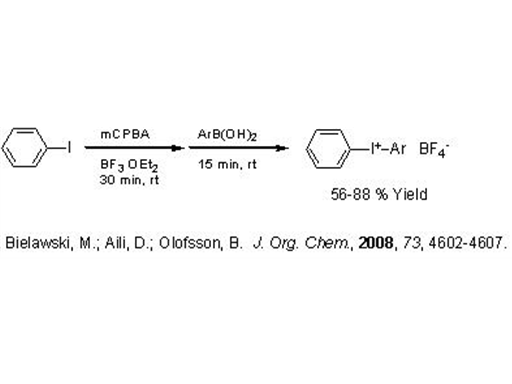
An interesting bit of chemistry was published by Berit Olofsson at Stockholm University in a recent JOC. The Olofsson lab has previously produced a method for the one-pot preparation of diaryliodonium triflates. This latest work provides diaryliodonium tetrafluoroborates (JOC, 2008, 73, 4602-4607).
The preparation of I(III) compounds usually starts with an Ar-I compound undergoing oxidation followed by an electrophilic addition/substitution to another arene. Regioselectivity is obtained by choosing a donor with a leaving group such as a boronic acid, stannane, or silane.
What is clever about this process is the fact that a BF4 salt is directly produced. Two equivalents of boron trifluoride etherate are used in the reaction which evidently results in some kind of disproportionation producing the BF4 counter-anion.
It is known that the reactivity of iodonium compounds is somewhat sensitive to the coordinating ability of the counter-anion, so BF4 is less undesirable than other choices (like chloride). Solubility is greatly influenced by the choice of counter-anion as well. This is particularly true in photo-initiator applications where the choice of carrier fluid may be limited.

Why stop at I(III)? I(VII) is 233% better! The most romantic hypervalent iodine compound must assuredly be fluxional structure I(CF3)_7 – and not just because it will be gaseous near room temp, MW = 609.946. Putatively from IF_7 re US Pat. 3992424, 395458 .
Berit min älskling, varför ville du att jag skulle sätta mig in detta?
🙂
Det här är inte mitt område what so ever ha, ha! Men kul att du gör väsen av dig och sluta inte att engera mig i allt du gör för den skull….
Mvh
Janne Herrström
Hello Jan, I’m sure your point is a good one (?), but since I don’t speak Scandahoovian it’s completely lost on me.