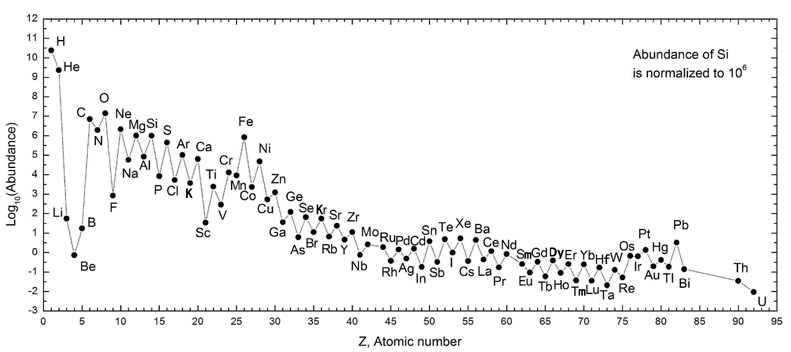
If you look the chart of elemental abundances in the cosmos below, you’ll notice that beyond hydrogen and helium atomic numbers, there is a steep log-scale drop in the abundances of lithium, beryllium and boron, or just ‘LiBeB’. This is followed immediately by a sharp exponential rise in the abundance of carbon, nitrogen and oxygen, etc. Beyond oxygen there is a general downward trend in elemental abundances. Well, except for iron, Fe. It is a special case related to the demise of stars. The zig-zag in the curve is explained by the Oddo-Harkins rule which postulates that even numbered atomic numbers are more abundant. I’ll leave this to the reader to explore.
Maybe 15 years back I decided to understand why each of the LiBeB elements are so scarce. And, if they are so bloody scarce, then how do they end up concentrated in ore bodies on earth? As a first-order approximation, you’d think that the explosion of stars and the resulting rapid dispersal of matter into the surrounding space would argue against the LiBeB concentrations on a planetary body like earth.
So, here is the question: Given that the cosmic abundances of lithium, beryllium and boron (LiBeB) are dramatically smaller than the succeeding light elements, how is it that “concentrated” ore bodies containing these elements exist on Earth?
As I look at it now, the answer is bloody obvious. But when I asked the question 15 years ago, my understanding of hydrothermal activity and fluid movement through the earth’s crust was pretty slim nonexistent. But hey, my focus in college was not astronomy or geology.
The graphic below shows how elements can be grouped according to a few particular categories. It is an absolute tragedy that the Platinum Group Metals (yellow shading) are in such the low abundance. These elements are uniquely valuable in chemical and industrial applications.
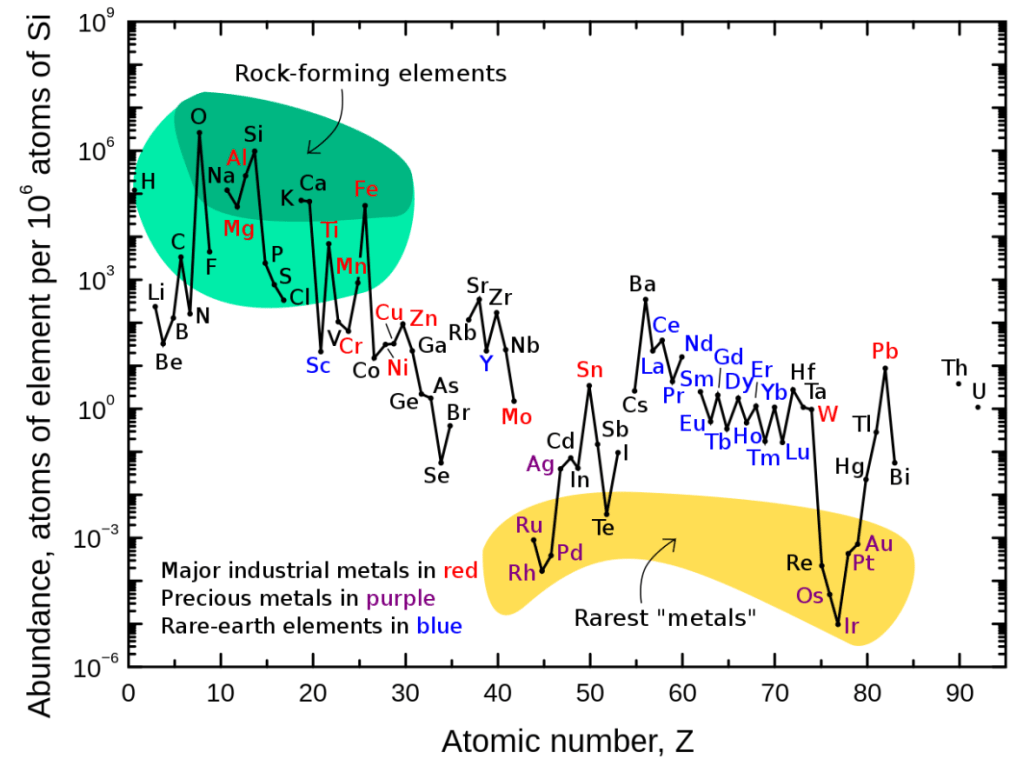
An even more fundamental question is, how is it that the cosmic abundances of lithium, beryllium, and boron are so low? Answer: they can’t survive the temperature and pressure conditions in the core of a star. They are fuels and therefore too delicate.
So LiBeB nuclei are produced during cataclysmic stellar explosions by some kind of spallation process, scattering fragments of larger nuclei into space. Another theory claims that cosmic radiation is responsible for the spallation of larger nuclei leading to LiBeB fragments. Subsequent generations of star formation resulting from an accumulation of hydrogen, helium, and heavier elements can lead to a star system with a protoplanetary disc where mass is brought into closer proximity. Mutual gravitational attraction between dust, chunks and aggregations of solid phase matter not already drawn into the star begin over time to aggregate and form planetary bodies. Some of the larger bodies are heated to liquid phase by collisions. Smaller mass objects may remain in the solid phase. Planet or moon sized bodies can collide to produce another planetary body or just debris. A fluid body of sufficient mass will spontaneously alter its shape in the direction of spherical such that all of the mass is as close to the center of gravity as possible. A spherical planet is one in which all of the mass is as close to the center of gravity with minimum potential energy as possible. A flat, disk-shaped body does not. It is hard to say just exactly how a flat planet-sized body would form with only gravity to drive it.
A cooling but still partially molten planetary body will begin to sort its large-scale composition by density and melting point. High melting point materials near a cooler surface will form solid crystalline bodies within the magma and then settle and possibly stratify according to density, then re-melt and disperse and convect to repeat the process. With cooling, the magma becomes increasingly viscous which would be expected to slow down mixing within the magma body. Over a long period, the planetary surface will continue to cool by loss of radiant energy and eventually the surface will crust over. Planetary bodies will accumulate mass by gravitational attraction as they sweep through space in their orbits, adding whatever chemical diversity that may be falling inwards to the surface. It seems reasonable to suppose that infalling dusts, rocks and asteroids are not uniform in their total compositions and may result in a non-uniform, spotty distribution of elements on the planet.
Solidification
Well before the surface cools to the point where a gas such as water can condense to form bodies of liquid water, water vapor in the atmosphere can convect to produce clouds at altitude, releasing latent heat and further adding to the heat transfer from the planet. Eventually, liquid water on the surface of a cooling planet can carry heat energy upward by conduction from the surface and atmospheric convection upwards, releasing the latent heat of condensation as clouds form in the upper atmosphere where it is colder. Heat transferred to the atmosphere will radiate infrared energy in all directions including space. The planet has developed weather.
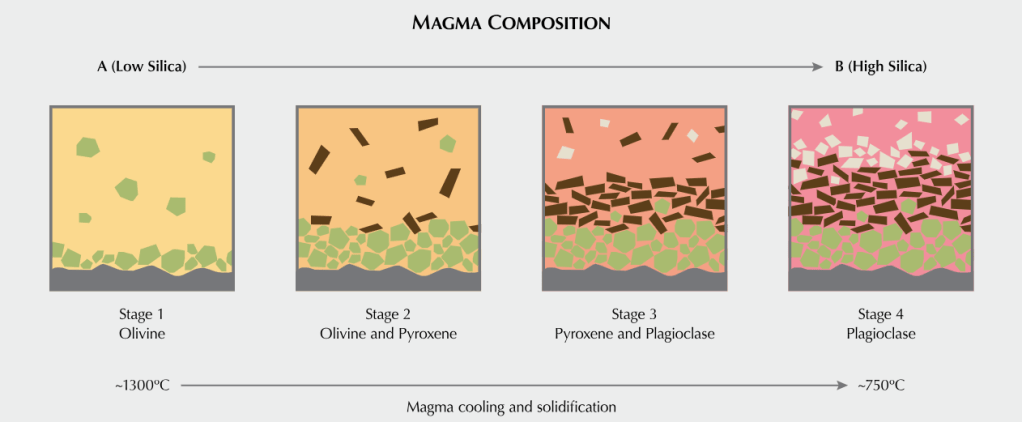
Solidification processes in a magma begin to spontaneously partition or nucleate into particular combinations of elements. Some groups elements like silicates combine with metals into molecules (covalent bonds and ion pairs). For a period of time on any given crystalline surface, the temperature will support equilibration to and from the magma wherein ionic species will attach to the crystalline surface from the magma. With sufficient temperature, ions can detach from the surface and diffuse into the magma. This process will continue as the temperature drops freezing the magma and suppressing diffusion.
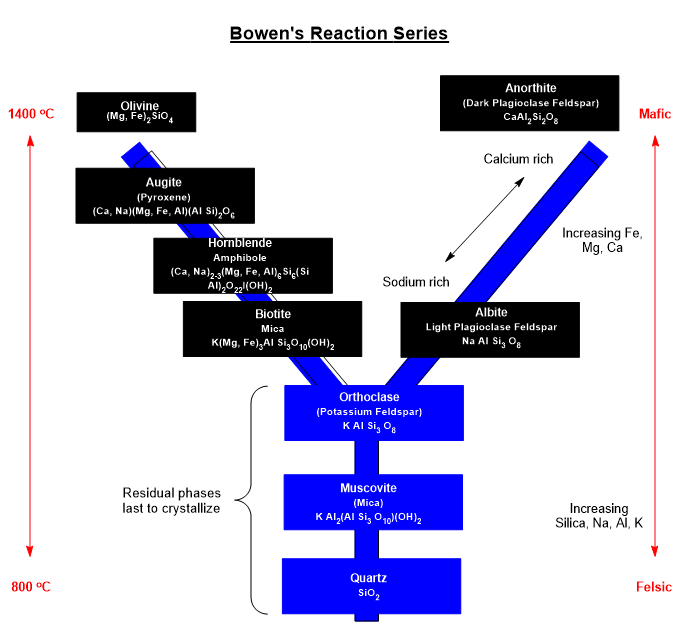
Bowen’s Reaction Series summarizes trends in the order of melting/freezing of various classes of minerals with temperature in magma. From location to location, the composition of magma is variable where some may be silicate rich and others silicate poor. That melting/freezing points of minerals vary with composition shouldn’t surprise my chemist friends.
Reaction selectivity is driven by the sign and magnitude of Gibbs energy in a particular transformation and the ability of the combination process to be reversed at the local temperature. The formation of crystals or their dissolution in hydrothermal fluids or magma is taken to be a type of reaction. Many of the species present at magma temperatures will be ion pairs.
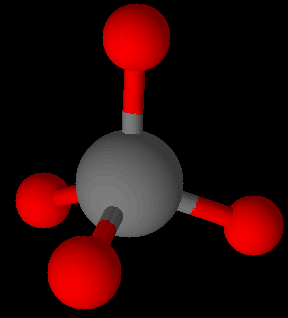
A few covalent species like the highly stable Si-O bonds in silicates are found in great abundance. The O of Si-O may or may not be connected to other silicon atoms such as … -O-Si-O-Si-O-Si-O-Si-O- … for examples. There is no upper limit to the number of O-Si groups possible in minerals, though their assembly can go many directions. Quartz is a 3-dimensional network homopolymer. All 4 of the oxygens on silicon are connected to adjacent silicon atoms via the oxygen linkages. Quartz is a mineral comprised of all covalent bonds. Quartz has a much higher softening temperature than manufactured glass.
Silicon readily forms tetrahedral connections with oxygen as in silicate (SiO4)4- units. Si-Si bonds are not found in nature.
In a hydrothermal fluid solution or in magma, ionic species randomly diffuse, collide or swap partners. Charged species like iron (2+) cations can collide but will bounce apart owing to their like positive charges. But at a sufficiently low temperature, a sulfide (2-) anion can collide with the oppositely charged iron (2+) cation and remain connected as iron sulfide. The two ions are now chemically bonded and in doing so, heat is released and dispersed into the surroundings leading to a localized increase in temperature. Heat spontaneously flows from high temperature material to lower temperature material. The localized heated surrounding crystallizing minerals can keep dispersing the energy outwards, but as it encounters more mass which also absorbs heat energy, the resulting temperature rise diminishes rapidly as the energy is diluted over more mass.
Chemical transformations have two broad drivers- kinetic and thermodynamic. Kinetically driven transformations favor the pathways that are the fastest Thermodynamic transformations are equilibrium-controlled meaning that transformations that are reversable will favor the end state having the lower Gibbs energy. The Gibbs energy (ΔG) combines the heat of reaction (enthalpy, H) minus the entropy (S) times the absolute temperature (T). The entropy accounts for whatever heat energy may be gained/lost from/to the environment. When a bond forming reaction occurs, heat is released and moves into the environment.
Mineral nucleation starts with a microscopic “seed crystal or compatible surface” that will provide a template to further crystal growth by like molecules. Some combinations of elements will begin to polymerize forming high melting point, low solubility molecules comprising silicates and aluminates.
The concentration of the Li or Be or B into what would later become economic ore bodies is driven by the flow of ground water. Subsurface hot water flow in the crust is referred to as hydrothermal flow and from it is where concentration of the LiBeB begins. An ore body is a mass or formation of rock that is enriched in some particular element or group of elements. Usually, these elements are part of chemical compounds rather than pure elements. Gold would be an exception.
Definition- Minerals and Rocks
Mineral– A solid substance with a well-defined chemical composition and characteristic crystal structure.
Rock– A solid, naturally occurring aggregation of minerals.
Water, and especially hot water under pressure, will chemically alter the rock it is in contact with. While it is in contact, some fraction of the altered rock will dissolve and some components may be entrained as suspended solids in the fluid. This will extract and partition part of the altered rock into a solution with a range of possible compositions depending on solubility, pH, temperature and pressure. In this way, elements get partitioned into solution phase and away from the solid phase.
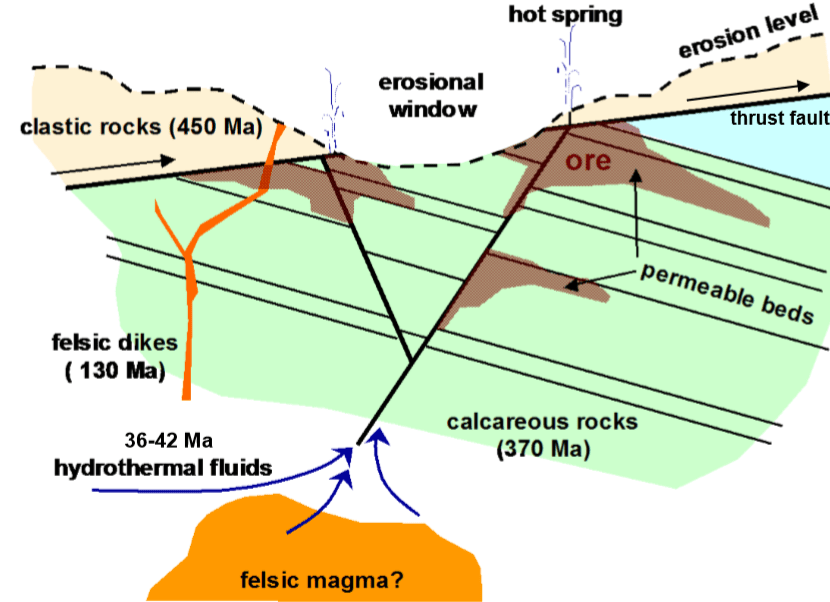
Hydrothermal solutions can sit in place for a very long time, eventually saturating with mineral components. If there is cooling in place, the saturated solution can precipitate to form a solid body of mineral filling the local spaces. The deeper the fluid, the warmer it will be and likely the slower it will cool. However, if the surrounding rock is porous or develops fractures or faults, the hydrothermal fluid will flow to the zone of lower pressure. If the flow is into a cooler zone, there may be precipitation of the least soluble components leading to solid mineral formation. Precipitation can also occur from a drop in pressure.
Solubility vs Temperature vs Pressure Example with Quartz
The effect of temperature and pressure on precipitation of silicate can be seen with the beautiful pressure/temperature/solubility relationship shown below. For instance, we can see that for temperatures at or below 150 oC, there is very little change in solubility of silicate with increasing pressure. On the higher side of the temperature scale at 350 oC, we see that there is a considerable solubility change with increasing pressure. At high pressure, say 700 MPa, the solubility of silicate is most sensitive to changing temperature.
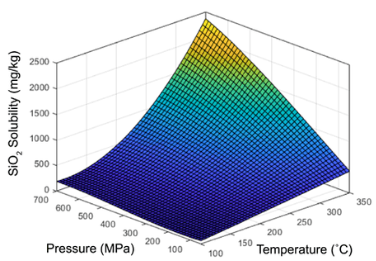
The research article above cites the solubility of SiO2 in mg/kg water, or ppm. I am using the term silicate indicating hydrated and charged SiO2, the SiO4 tetrahedral form, which is known to dissolve in water. It is common in some of the geological literature to refer to the neutral oxide form of a mineral or metal.
SiO2 is often observed in its amorphous or crystalline topological polymer (or network polymer) form as a quartz vein, pegmatite or crystalline mineral component of a felsic igneous rock. Quartz found in a vein is there because a fault or fracture was available for filling with a hydrothermal fluid. As the curve above infers, saturation and precipitation can be abrupt with a sudden drop in pressure during a fault movement or sudden opening of a fracture network. Quartz veins are often accompanied by metallic gold in the upper oxidized zones (gossan) of the formation. In the context of mining, these veins are sometimes called quartz reefs.
Back to LiBeB
Although LiBeB elements are scarce, there are hydrothermal processes in the crust that can concentrate them in economic quantities.
An excursion into ore geology shows that selective hydrothermal extraction and transport are critical to the formation of a great many types of ore bodies. In fact, all three elements of LiBeB are moved by hydrothermal fluid transport on Earth at some point.
Lithium
Lithium metal is quite reactive and not found on Earth in the neutral metallic state. It is a single-electron donating Group 1 element and the lightest atomic weight metal of all the elements. Lithium has a large standard reduction potential of -3.05 Volts and is an excellent donor of electrons and a poor acceptor of electrons.
Lithium metal reacts with three major components of air: water, to form LiOH + H2; Oxygen to form Li2O + Li2O2 + H2; and nitrogen gas to form NLi3, lithium nitride. While other air-reactive metals can be stored under a hydrocarbon like kerosene for protection, lithium will float in these liquids and may be mixed with a heavy hydrocarbon or grease to keep it covered.
Lithium deposits can be split into two general domains- brines/muds/leachates, and hard rock deposits. Spodumene is the common hard rock source of lithium found in a few places around the world. Spodumene is lithium aluminum inosilicate and has a few variants such as Hiddenite, Kunzite, and Triphane coming from trace elements present in the surrounding rock.
The Silicate Zoo section is a bonus feature and may be skipped.
Bonus: A Step into the Zoo of Silicates
Spodumene is an inosilicate. Silicates as a group make up 90 % of the Earth’s crust. The basic unit is the tetrahedral [SiO4]4- silicate with 4 oxygen corners of the tetrahedron. These are known as ortho- or nesosilicates. The 4 minus charges are balanced by 4 plus charges provided by one or several metal cations at a time. Orthosilicates are the simplest variants of silicates.
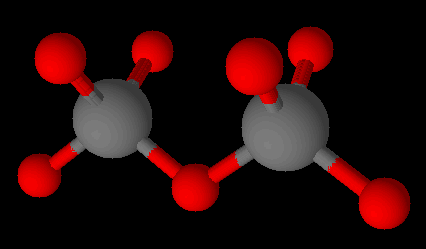
The corner oxygen ions of silicate can be attached to the silicon of another silicate. These are called the sorosilicates and have the formula [Si2O7]6−.
Silicate units can also form rings called cyclosilicates. The rings consist of silicate tetrahedrons with alternating linkages of silicon-oxygen atoms. The general formula is [SinO3n]2n−.
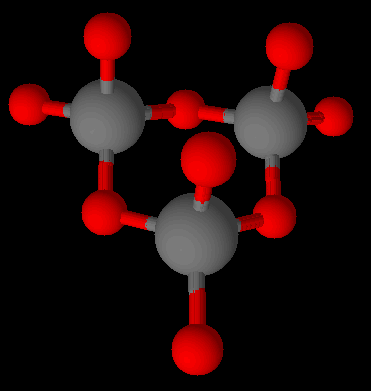
There are several varieties of inosilicates. As a group they link silicate units into interlocking chains. There can be a single chain (pyroxenes such as spodumene) or two (amphiboles such as asbestos). The single chain inosilicates have the formula [SinO3n]2n−. The double chain inosilicates have the formula [Si4nO11n]6n−.
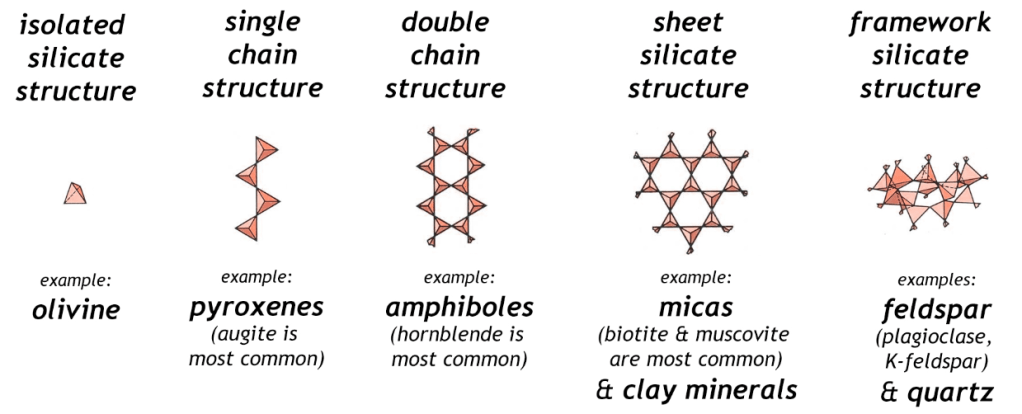
Phyllosilicates are sheet structures of silicate and have the general formula [Si2nO5n]2n−. This group includes micas and clays.
The tectosilicates are 3-dimensional frameworks and have the general formula [AlxSiyO(2x+2y)]x−. Note that aluminum may be present. This tectosilicate group includes quartz, feldspars and zeolites.
If you look at all of the general formulas, note that the negative charges must be balanced with positive ions (cations) of some kind. They are typically metal cations that vary in positive charge and ionic radius, although they can be capped off with hydrogen. A metal cation with its positive charge and ionic radius can be most easily replaced by a different metal with the same charge and similar ionic radius. Different charges and ionic radii are possible replacements but cause distortions in the lattice structure.
Minerals can form such that a silicate [SiO4]4- unit can be replaced by one or more aluminate (AlO4)-4 units and the metal counterions can be substituted by other metals of the same charge and similar ionic radius.
Back to Lithium
A growing fraction of lithium exploration and production involves lithium brines. Subsurface brines enriched in lithium (up to 0.14 %) can be pumped to the surface and exposed to sunlight in large evaporation ponds. Brines are saline solutions comprised of numerous soluble ionic substances. The lithium component must be isolated to a specified level of purity prior to sale. The most common product leaving a lithium mining operation is the insoluble lithium carbonate.
The process of concentrating a mineral from the ore is called beneficiation. An ore is comprised of the target mineral and gangue or mine waste from previous activity. The idea is to use economical physical and chemical methods to remove the gangue from the target mineral.
The largest collection of lithium brine reservoirs, known as salars, is in the Lithium Triangle in Latin America. The countries in the Triangle are Chili, Bolivia and Argentina. This area holds more than 75 % of the world’s lithium resources under their salt flats.
The salar brine is pumped into an evaporation pond and allowed to concentrate in the sunlight for a year +/-, then it is filtered and sent to another evaporation pond for further concentration. What happens next depends on which of the several possible processes is being used. The type of processing that is used depends on few things: The composition of the ore and interfering substances present; the company may have in-house technology they can adapt; there may be patent constraints in force; the process economics will apply considerable effects on equipment size and required annual throughput.
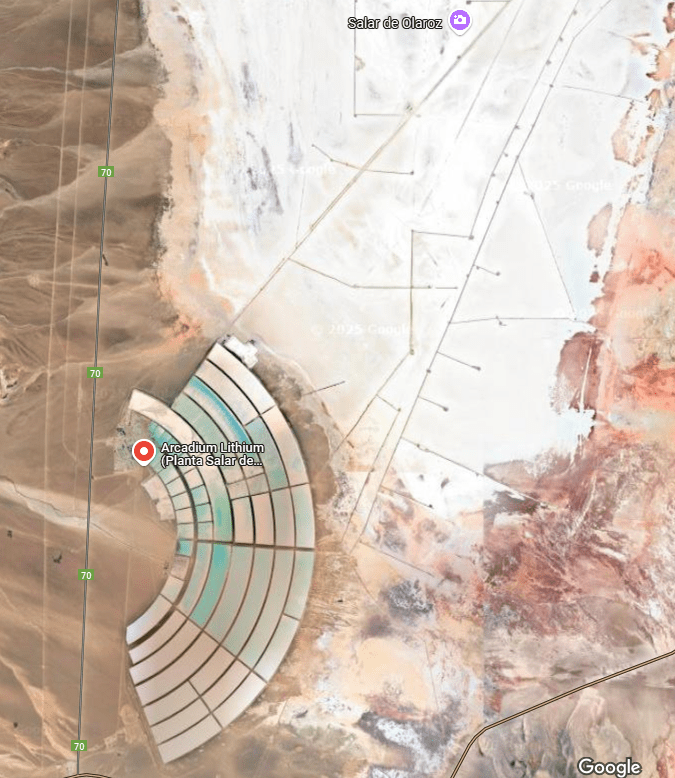
Economic ore is that resource which can be beneficiated and further refined and sold at a profit. So, the amount of ore that constitutes an economic deposit can shrink or grow with the market price of the product.
A recent development in lithium resources is the Rhyolite Ridge Lithium-Boron Mining Project near the ghost town of Rhyolite, Nevada, and southwest of Tonopah. The mine will be a large scale open pit operation operated by the Australian mining company ioneer. The mining will be carried out in the conventional drill-and-blast, and load-and-haul method. In the beginning the mining fleet will use automated haul trucks.
Even though nature has provided a concentrated lithium ore body, people still have to go to considerable lengths to produce economical lithium product, all the while gambling on the market price in the future.
Beryllium
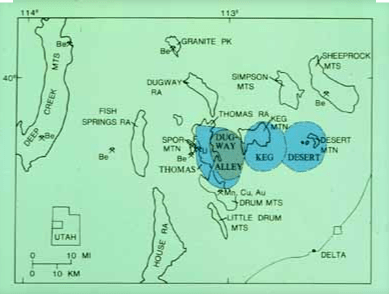
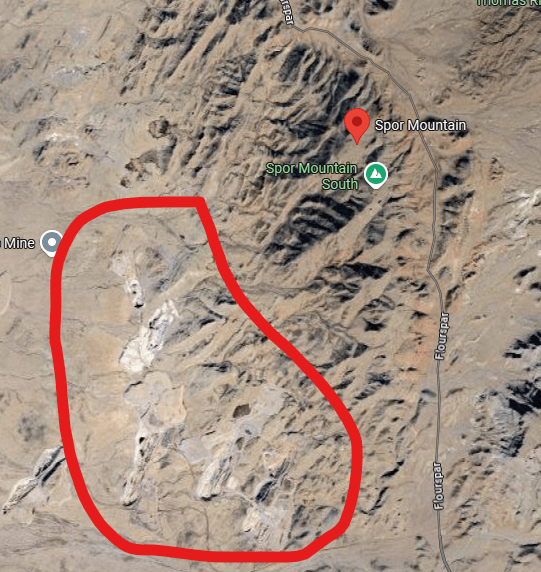
Beryllium, Be, atomic number 4, has some very useful properties that make it a valuable metal. It is also quite scarce. The world’s richest beryllium deposit is located near Spor Mountain in Utah. The mine is operated by Materion Brush Inc., formerly known as Brush Wellman. The mine is operated by Materion Inc., formerly known as Brush Wellman. The beryllium rich tuff is located by trenching and drilling. Tuff is compacted volcanic ash that has been cemented into porous rock by contact with water flows. The area also contains fluorspar and uranium, separately, in combination with beryllium. Bertrandite (Be4Si2O7(OH)2) and beryl (Be3Al2Si6O18) are the chief beryllium-bearing minerals and fluorspar is a common accessory mineral.

Spor Mountain is located in South Western Utah in basin & range country that extends across Nevada to California.
The above map is from the US Geological Survey and shows 3 extinct calderas in the immediate Spor Mountain vicinity represented in blue. It is interesting that tuff, made from pyroclastic flows and ash where particulates are later cemented together, is the rock in which beryllium is found in the area AND there are 3- count ’em 3 -calderas right nearby. Coincidence?
Beryllium is a rare element on earth showing up in veins, pegmatites and tuff. In the cosmos its existence is thought to be due to cosmic ray spallation.
“The beryllium tuff is a favorable host for beryllium ore because 1) it is adjacent to faults and rhyolite vents where mineralizing fluids could enter the tuff, 2) it is a porous, reactive conduit for mineralizing fluids, including both hydrothermal and ground waters, and 3) it contains carbonate clasts, which reacted with fluorine-rich fluids to precipitate fluorite and beryllium.” Source: USGS, 1998

Boron
We’ll defer to the Wikipedia to provide background information on the element boron. The main source of economic boron is borax. Borax is a type of evaporite originating from hydrothermal fluids that have flowed to the surface where subsequently the water carrier evaporated to afford dissolved and suspended solid borax, a hydrated borate. Boron the pure element is not found, it only appears as set of various borates. There are monoborates, nesoborates, inoborates, phylloborates and tektoborates. The prefixes below are applicable to silicates and other oxides as well. The older Nickel-Strunz categorization system has been used to describe the classification of complex borate anions found in minerals.
- neso-: insular (from Greek νῆσος nêsos, “island”)
- soro-: grouped (from Greek σωρός sōrós, “heap, pile, mound”)
- cyclo-: rings of (from Greek κύκλος kúklos, “circle”)
- ino-: chained (from Greek ίνα ína, “fibre”, [from Ancient Greek ἴς])
- phyllo-: sheets of (from Greek φῠ́λλον phúllon, “leaf”)
- tekto-: three-dimensional framework (from Greek τεκτονικός tektōnikós, “of building”)
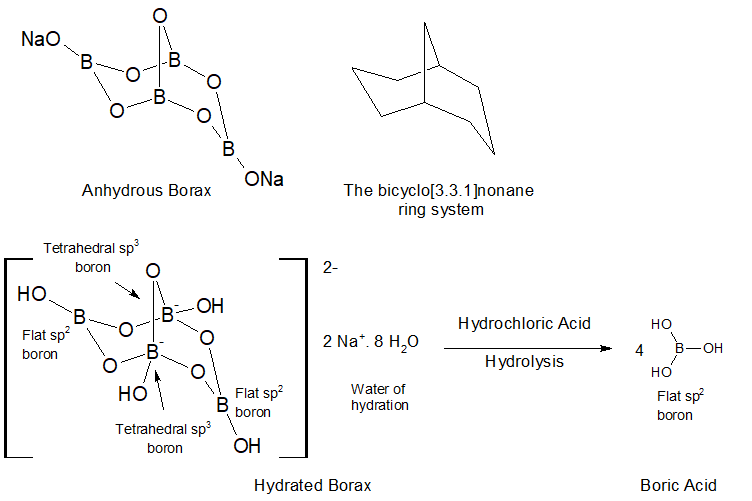
The structures of borax and hydrated borax above have been reduced to their minimum hydration. In reality these are idealized structures not reflecting the agglomeration into larger hydrogen bonded structures. Neutral sp2 boron atoms have an empty, low-lying p-orbital which can bond to the O of O-H groups whether from water or other borates.
The actual composition of any oxy-borate including borax will depend on its thermal history and its chemical environment. Attempting to dehydrate a borate may indeed remove water, but in doing so may open up a p-orbital on boron that would allow an exchange of oxygen species, whether another borate, boric acid or just another water molecule. This is not worrisome when producing boric acid, but when one of the OH groups of boric acid is replaced with a carbon atom, then dimerization and trimerization may occur even at low temperature under vacuum to form boronic anhydrides.
When ordering an organoboronic acid, look at the specs very carefully. Sometimes the water spec may be quite broad due to adventitious dehydration. Using azeotropic removal of water will work by toluene azeotropic distillation, but you may not recognize the 1H-NMR spectrum of the toluene solution because this dehydration method may produce the dimers, trimers and oligomers from the original organoborate. In my experience, the anhydrides work very well in the Suzuki coupling.
A trial sample of an organoborate must represent what can actually be made at scale. A lab sample will have an analysis of what a skilled chemist can do under optimal conditions. If the customer wants bulk boronic acid matching the qualifying lab sample, then you may be sunk if you cannot reproduce the sample specs in the plant. Been der, done dat.
When submitting an organoboronic acid to a customer for approval, avoid sending the very best material from the R&D lab. They will spec on that sample and expect to see the same thing from a production run.
In the organic synthesis area that I have been witness to, boron showed up in our lives mostly for purposes of aryl coupling chemistry with an aryl halide, an organoboronic ester and a palladium catalyst. The nomenclature gets confusing with boron species. Borax is a borate because of the B-O-B and B-OH bonds. But KBF4, potassium tetrafluoroborate, has a boron-based anion with 4 fluorides. The -ate suffix does not always mean that oxygen is in the formula. Many anionic boron species have a net negative charge because a group like OH or F brought the negative charge to make a tetrahedral boron. Quaternary borates are often used as a weakly coordinating conjugate anions where non-interference by the anion is needed.
Lithium, beryllium and boron are all three transported by meteoric and hydrothermal water flows. When hot, pressurized water penetrates a fault or fracture system, the fluid can interact with whatever rock it is in contact with. Over long periods of time the fluid can corrode the wall minerals of the fault.
In this scenario, water can do several things to the rock. At the atomic level, the outermost layers of a crystal lattice in the mineral may be subject to aqueous hydrolysis making metal hydroxides, MOH, and liberating the anionic X– species from the latticework. Or, the water may just pull unaltered polar species into solution where they can swap ionic partners and either remain in solution or precipitate onto the surface features of the rock. If the hydrothermal fluids are in motion upwards to cooler regions above, precipitation may occur as the fluid naturally cools and depressurizing. Over time, layers of different insoluble material can stack onto the previous layers. This can go on until the void in the rock is filled and can no longer pass fluids through the fractured formation.
Conclusion
Despite the cosmic scarcity of each of the LiBeB elements, these elements may be found on or near the surface of the Earth concentrated as minerals in an ore body. Transport and placement of enriched individual LiBeB ores derives from multiple instances of differential solubility from the source rock to the ore body. Minerals that have more water solubility than adjacent minerals will tend to be transported in aqueous flows. The transported mineral gets concentrated in this way, but so do the minerals left behind. Aqueous flows at the surface can drop out their dissolved minerals as the surface water evaporates. As the surface water concentrates and cools, a sorting process is underway driven by solubility properties.
The original salt ion pair, M+X–, may be extracted into in water and dissociated to produce M+ and X– ions in solution. Other ionic substances like N+ and Y– can switch partners in a double displacement reaction and produce MY and NX. If it turns out that NX has poorer solubility than MY, then the mixture of M+X– and N+Y– will produce a precipitate of NX, leaving much or most of M+Y– in the water. Minerals deposited from evaporation are called evaporites.
Which is more desirable from the manufacturing perspective, the precipitate or the concentrated solution? Not being a mining engineer or an economic geologist, I can only speak as a person from the fine chemical industry. The precipitation of the desired product from a complex mixture seems a bit more desirable in that it is both an isolation and purification process. Often the solids can be washed and dried in a filter dryer and bang, you have your product.
Precipitating out an undesired solid component from a mixture, pure or not, while leaving the desired product in solution with solvent and other side components from the reaction is a bit less desirable. This version is at least another step away. If the product is a solid, perhaps a scheme can be found for precipitating it as well.