Summary:
What can a chemist possibly have to say that could be even marginally interesting about extraterrestrial life or evolution? Well, as far as extraterrestrial life and the search for it goes, I would say that all of the metallurgy, semiconductor fabrication, liquid hydrocarbon fuels, chemicals, transportation technology or polymers exploited in radio or optical astronomy, have some element of chemistry in their manufacture.
………………..
The quest to discover life beyond Earth captivates many in the broad field of space science. The Search for Extraterrestrial Intelligence (SETI) has played a significant role in astronomy and space science communities. However, the search extends beyond intelligent life; any form of life or even the essential components and conditions conducive to life, are of keen interest.
It is widely accepted that the physics governing our planet and solar system likely applies universally. While this is a hypothesis, it is a reasonable one. If the physics are consistent, then the chemistry should be as well. Consequently, the behavior and limitations associated with matter would be uniform across the cosmos. This reasoning suggests that life elsewhere in the universe would be governed by the same chemical and quantum mechanical principles familiar to us.
The “Anthropic Principle” has caused much debate, with Wikipedia noting that “Anthropic reasoning is often employed to address the notion that the universe appears to be precisely calibrated for life.” The mystery of why numerous physical constants and their ratios needed to be exactly as they are for life to emerge on Earth has intrigued many.
To say the Big Bang’s initial pressure and temperature were high is an understatement. As the universe expanded and cooled, energy barriers emerged that shaped the interactions of matter and of photons, placing boundaries on the spontaneous transformation behavior of matter. Pathways of interaction emerged, steering transformations towards increasingly specific outcomes. Essentially, it’s basic kinetics: the quickest transformations and their stable products start to prevail and fill the universe.
If physical constants are emergent at the moment of the Big Bang and become manifest down the timeline, could it be that another Big Bang could happen that is not conducive to life? There would be nobody there to ponder these questions. Life is here because it was possible and maybe even likely here and there.
The phrase “finely tuned for the existence of life” seems to leave open a crack for a creationist view. Absent the many spooky bronze and iron-age theories still in practice today, naturally a sentient being can look at her/his/its existence and marvel at how beautifully synchronized and proportioned the machinery of the universe is. Certainly, there must be a hidden message in this, right?
ET? What th’ …?
Animals like mammals, birds, fish, and even some invertebrates like octopi and crabs are considered to be sentient. According to Google, sentient animals are those that can experience feelings and sensations, both positive and negative, like pleasure, pain, joy, and fear. So, while an octopus may have elements of sentience, could distant observers elsewhere in the galaxy detect them from optical or radio astronomy techniques? Try as it might, the ability of an octopus to construct a powerful radio transmitter and beam a message into the cosmos is sorely limited by its physical anatomy. Except for humans, no other sentient life form on Earth is known to construct a radio transmitter that would serve as a beacon of sentient life.
Until recent history, SETI was limited by the lack of technology to light up the universe with our own signals or to detect faint manufactured signals across interstellar space. At such point that metallurgy, electrical engineering and the hundreds of other critical and apex technologies bloomed into a sufficient state of development, no intelligent emanations from Earth found their way into space.
While TV and radio broadcasts began their journey into space, it is important to realize that our signals were encoded onto carrier waves. Amplitude modulation (AM) signals carry their information by simply varying the magnitude of a single frequency in time with the human voice or music. This is most likely to be grasped by alien radio astronomers. Frequency modulation (FM) is a bit more challenging because audio signal is mixed with a carrier signal by a heterodyne circuit. Extracting useful information would require them to pull audio frequency information from the heterodyned signal.
Television is much more difficult. While the alien radio astronomers may have figured out FM encoded radio information, the particular details of the TV raster scan are based how human engineers decided to interlace and sequence scans to produce an image on a screen of a particular aspect ratio. TV designers took advantage of the human’s persistence of vision to seamlessly follow moving pictures to give continuous images yet maintaining a fast enough frame rate to avoid flickering. The television’s electronic timing is based on frame rate, the number of interlaced lines, and the aspect ratio of the screen.
The point of this TV discussion is that a TV signal must be deconvoluted into a signal that properly displays an image and plays the sound on a particular piece of equipment. This could be challenging for an alien radio astronomy research group to decode.
All of this talk about an octopus developing radio astronomy presupposes that its unique octopussian sentience includes such desires.
It could be that the initial energy at t = 0 yielding the primordial plasma constituting the early Big Bang was only capable of producing a specific set of fields producing elementary particles which then give way to a specific set of quantitative relationships and properties. The burst of energy causing the Big Bang must have had constraints driving its transformation into matter, which is also constrained by quantum mechanics, etc. Maybe the present universe is simply what primordial energy naturally does when expanding as a universe. Why do the quantitative values of physical constants need to be variable? An imaginary and feverish conundrum.
As the highly energized primordial plasma of the Big Bang began to cool, matter and energy channeled into particular states. The particle energy states that had the highest barriers coalesced first followed by subsequent lower energy plasma condensing into other particles. I’m drawing a crude analogy to the process where individual minerals form from cooling magma according to their melting points.
There is a notion prevalent among Creationists that the probability of a life form spontaneously forming from individual atoms is 1 in 10large or some other inconceivably miniscule chance. And if that was how life had to form, then the Earth would still be a sterile wet rock. But that is not how chemical transformations work.
Central to the Creationist view is that evolution cannot happen because there is nothing but random chance to guide the molecules of life into a highly complex organism. They start with the assumption that life arose purely from random chance. I hope to show that this assumption is false.
All atoms and molecules have properties that either qualify or disqualify them as a candidate for a given atomic or molecular transformation. All molecules have properties that either qualify or disqualify them to take part in a transformation resulting in a given product. The words “qualify” or “disqualify” could mean that something will or will not happen absolutely. But just as likely, the words could mean that a transformation is just too slow at a given temperature to give the desired effect. As it happens, temperature is critically important to molecular transformations. At a low enough temperature, most transformations will slow to a negligeable rate, shutting down that particular transformation channel. In general, where there are competing transformation channels, the fastest channel will prevail in producing its product.
All molecules have a limited set of reaction channels at a given temperature as a result of their particular reactivity.
What we think of as ‘ordinary’ chemistry is more precisely the electronic behavior of valence electrons. Nuclear chemistry also exists but in the domain of nuclear change.
Valence electrons on earth will behave the same everywhere in comparable conditions. Chemistry happens at the outer, valence level of ions, atoms and molecules. So, we should expect that bond forming and bond breaking mechanisms should be the same throughout. All of this leads to the high likelihood that chemical reaction mechanisms elsewhere in the universe should not be unfamiliar to Earthlings in general.
Life on earth exists as a result of the behavior of particular chemical substances within a range of chemical and thermal environments. The range of chemical environments and substances present during the initiation of life is thought to be quite different than what we find on earth at the present time. For instance, gas phase molecular oxygen was not present until a considerable time after life began. The initiation of life on earth was under anaerobic conditions and was able to start and survive with the materials at hand. Biochemistry is a series of reduction/oxidation events driven by the Gibbs energy of a transformation as is all of chemistry. Even on anoxic earth, diverse oxidizers were present.
Today, anaerobes are known to use the oxidative properties of inorganic species like sulfate (SO42-), nitrate (NO3-), ferric iron (Fe3+), carbon dioxide (CO2) and manganese (Mn 4+). Other anaerobic oxidants include chromate (CrO42-) and arsenate (AsO43-) which may have been present as well. Reductants include nitrite (NO2-), ferrous iron (Fe2+), and sulfide (S2-).
Oxygen is the third most abundant element in the universe and the second most abundant heavy element on earth behind iron. Many elements are strongly attracted to the abundant oxygen so it is no wonder that so many minerals are oxides of one sort or another. Oxyanions like silicates, carbonates, sulfate, nitrate, and oxides like CO2 or any number of metal oxides all contain oxygen that has been bound with another element. The oxygen pulls negative charge away from the central element making it electron deficient. In the case of sulfate and others, the actual oxidizing part is the atom with the oxygens attached, in this case the sulfur.
The presence of life on Earth means that there is a “habitable zone in parameter space“. All of the parameters affecting biochemistry must align in such a way that a zone of allowable chemical and physical conditions will exist. Many things must exist simultaneously such as the many properties and abundances of chemical substances, a suitable atmospheric composition and pressure, a planetary temperature range allowing for the presence of liquid water and the presence of sufficiently reactive organic molecules.
Not every transformation of matter is within reach in a given condition. Chemical reactivity which comprises kinetics and thermodynamics has the effect of channeling matter into a finite number of probable pathways. This bestows the property of selectivity. For any given chemical substance, only a certain limited group of transformations are possible or likely, given the conditions.
Life as we know it exists because our biomolecules were robust enough to survive their chemical and thermal environments, but not so robust that they resist the needed transformations. Life depends on biomolecules being moderately stable but not by too much. Biomolecules can organize into particular structures that are physically robust, like the chitin shells on shellfish. In the chemistry of life, chemical transformations must be tolerant of the aqueous environment in and around an organism, but not so tolerant that the necessary reactions are too slow or too fast within the narrow range of environmental temperatures available.
Organisms on earth are tolerant of water at the level of molecules. The internal apparatus of the cell is an aqueous environment having some amount of viscosity. In order for molecules to interact, they must collide with each other. Life in the solid phase would mean that biomolecules would be immobilized and unable to collide and react. Cell structure for metabolism and reproduction would not be feasible. Life in the gas phase is limited by the vapor pressure of the necessary substances. Many, if not most, biomolecules would not tolerate the heat necessary to volatilize. They would decompose.
A diversion into molecular evolution.
I’ll just blurt it out- ongoing evolution requires heritable change in a genome. A genetic change must be survivable for the parent cell to reproduce and produce viable daughter cells. The inherited mutation must not be deleterious to further reproductions of the subsequent generations. A mutation may randomly result in something that has either a lethal effect, no effect, or produces some biomolecular improvement. The mutation may be as modest as an enzyme alteration causing it to bind either more or less tightly to a ligand resulting in a few percent change in rate of some the enzyme’s function. This could translate into better efficiency in producing some cell structure or better use of energy. It could also be that nothing changes as a result of the efficiency alteration, or that it has an overall negative effect further challenging the survival of the cell line in a nonlethal way.
There are two kinds of changes that can occur with DNA. One is a change in the sequence of the DNA molecule itself. The other kind is “epigenetic” which is heritance not reliant on changes on the DNA sequence.
Creationists like to make a show of the probability of random chance producing even simple ordered sequences as fantastically small. Actually, their superficial analysis of permutations and probability looks plausible. I can’t argue with the low probability of individual atoms coming together randomly to form a living organism all at once. However, the beginning assumptions are wrong. Life did not spontaneously form out of a bunch of loose atoms by simply condensing into a centipede or a human. Change in evolution happens at the molecular level a step at a time. A change in the amino acid sequence of any given enzyme must trace back to a change in the DNA sequence to pass along a heritable mutation. Evolution moves by fits and starts. A mutation may have no effect, advantageous effect or deadly effect.
At the level of molecules, change happens through very definite chemical mechanisms. Molecules are constrained to do certain things and in a particular way. It’s like a channel. Sometimes two or more channels may be possible. In this case, the fastest channel will dominate in output and influence. An evolutionary change might cause a biochemical transformation to stop, speed up, slow down, or be more or less specific in outcome.
Molecular bonds vibrate in the range of 1013 to 1014 Hertz. A hydrogen molecule will reportedly undergo 2.5 x 1010 collisions per second at 2 bar and 24 oC. If two atoms or molecules are to react, then they must collide. At a given temperature, a collection of hydrogen atoms will be dispersed over a statistical distribution of energies.
Biochemistry on earth has evolved around water and takes advantage of certain properties of water. Its ability to hydrogen-bond is exploited extensively in biomolecule structures. Water has the ability to accommodate charged species or neutral dipolar species. This is called hydrophilicity. It is important not just to keep ions and molecules in solution, but also to stabilize the transition of a reaction if it generates a momentary dipole.
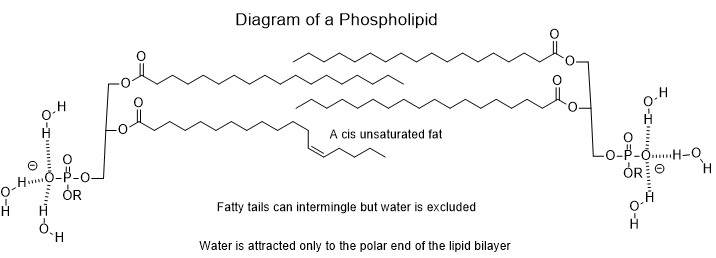
Water is immiscible with substances having a large hydrocarbon protuberances like fatty acids, phospholipids or certain side groups found on a few amino acids. This is called hydrophobicity. Terrestrial biochemistry exploits both hydrophilicity and hydrophobicity.
A benefit of hydrophobicity in biochemistry is that fatty substances like phospholipids will spontaneously organize into the lipid bilayer structure. Hydrophobicity in this case leads to the formation of stable compartmental structures. Life takes full advantage of the lipid bilayer in the production of the cell wall. This keeps all of the necessary biomolecules contained and concentrated for effective and timely biochemical transformations to occur. The cell wall excludes a great many deleterious substances as well. However, many protein structures have sections that are sufficiently hydrophobic as to be compatible with the hydrophobic lipid part of the bilayer. This property allows the protein to anchor itself within the bilayer leaving the more hydrophilic portions of the protein jutting out into the extra- and intracellular aqueous media. Many of these proteins penetrating the bilayer- channel proteins- are sufficiently hollow as to allow ions or molecules to pass through. Even better, the ability to pass ions or molecules through can be switched on or off by other biomolecules or with drugs.
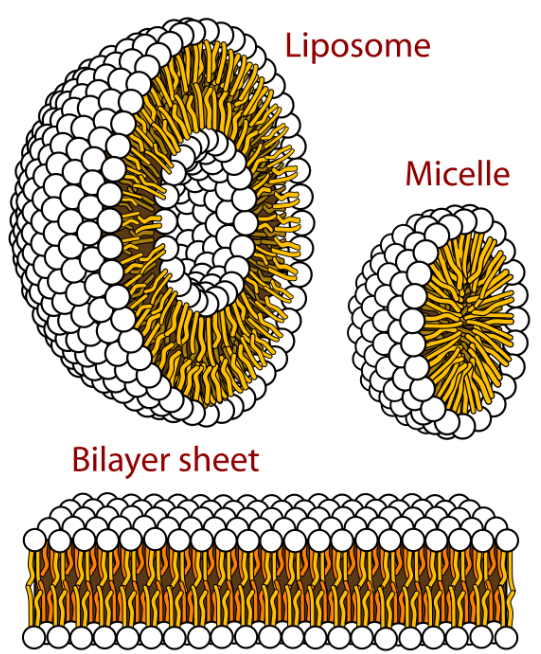
Cells have compartmentalization and cell walls simply because of the incompatibility of the polar water molecule and nonpolar hydrocarbons. These two incompatible liquids arrange in a way that minimizes the surface area of contact between them. They will form layers when stationary or droplets when one is dispersed in the other. This is the minimum energy condition they spontaneously go to. Micelles will even form spontaneously in your soapy dishwater.
Life on earth presently requires many environmental conditions to be just right. Cells of micellar-like construction take advantage of the hydrophobicity of substances with long chain hydrocarbon parts on one end and charged or polar features on the other side. Micelles are structures that spontaneously form in water. Living cells adopt a bilayer structure based upon the tendency for “likes to dissolve likes.” That is, non-polar hydrocarbon features “prefer” not to be in contact with polarized water, but rather cluster in a way that minimizes water-hydrocarbon surface contact. The effect of carbon chain structures in the biochemistry of earth is the stability of carbon-based structures and the wide variety features it can accommodate. These features include stable carbon-carbon chains as well as carbon bonds to hydrogen (H), nitrogen (N), oxygen (O) and sulfur (S) in particular. Carbon is unique in that it readily allows the formation of stable double bonds with itself or N, O, or S. Carbon also can form triple bonds with itself or N. Cyanide and acetylene are examples. The ease and stability of carbon bonded to C, N, O and S, along with the stability of multiple bonds on carbon all point to it as an excellent candidate for as the ideal building block for biomolecules.
It is often mentioned that since silicon has certain similarities to carbon why isn’t life based on it? Silicon-silicon bonds are prone to oxidation and not found in nature. Silicon is almost always found in nature as silicate in its various forms in minerals and very often in variety of silicate oligomers and polymers. Silicon-nitrogen and silicon-sulfur substances are not easy energetically. Furthermore, silicon does not form double bonds with itself or other elements. So, the variety of structural motifs silicon can form isn’t as broad as carbon. Silicon vastly prefers to be silicate in nature. Silicon is not found in biomolecules despite its high abundance in the nature.
Conclusion
I’m trying to make the point that extraterrestrial life will surely be different from life on earth at the macroscopic scale but maybe not so much at the level of molecular transformations. Every living species today trails behind it a unique evolutionary history, some of which remains in their genomes. Despite the huge variety of life forms on earth and all of the attendant structural variability that goes with it, we all share the use of DNA/RNA, proteins, carbohydrates, phosphates, lipids, calcium, magnesium, potassium, sodium, etc. All life forms on earth are able to capture and use energy as well as reproduce.
The history of life reveals an obstacle course through which organisms struggled to stay alive. Those that did survive had no way to anticipate the future and no way to prepare for it even if they were able to “anticipate” at all. The history of life is the history of challenges to survival.
Humans exist today because our ancestors going back into deep time were able to survive both anaerobic and oxygenated earth, snowball earth, competitive pressures from other life forms, vulcanism, cometary impact, solar UV radiation, chemical toxicity from the environment, disease and climate.
Today we can add stupidity to the list of survival challenges. Can we survive the results of our behavior? Humans have a brilliant streak in developing weapons- explosives, guns, nuclear, biological and chemical weapons. If all else fails, there will always be the sharp stick and club.
Humans are the way we are because of the way that natural history unfolded. A planet with the same makeup and conditions 3 billion years ago would evolve life in a different way than we went. Evolution happens because of the ability of our genetic material to be just a bit unstable and to be passed on in reproduction. But this change is a random process in both features and time. A genetic change can be fatal or helpful. The manner and schedule in which random genetic alterations happen is impossible to predict. Evolution is blind going forward. Another try at evolution is highly unlikely to produce Homo sapiens again.
Any given “intelligent” species may or may not invent or use radio technology. Therefore, they may or may not emit or receive radio transmissions. Such creatures would be undetectable using radio astronomy. Although two patents for wireless telegraphy came out in 1872, humans have only had useable wireless telegraphy since 1895 (Marconi). As of this writing, only 128 years have elapsed since Marconi sent his first long distance (1.5 mile) radio communication.
We have only had radio communications for 128 years in the entire history of our species. In order to have this invention in 1895, the European enlightenment had to happen leading to the idea of scientific inquiry and a minimum understanding of physics and chemistry. The voltaic pile had to be invented which gave way to further refinement of electricity. At minimum, the metallurgy of iron, copper and zinc (for brass) had to be in place for the for the discovery and use of electricity. The path to broadcasting and receiving radio waves required a fair degree of curiosity and industrialization.
