C&EN has a web page devoted to a linked bibliography of safety-related letters to the editor. It is worth having a look at. It is good to have a healthy interest in energetic reactions and incompatible substances.
Category Archives: Chemistry Blogs
A critique on scale-up suitability
In my quest to stimulate bench chemists to think like industrialists, I like to bring examples of chemistry from the literature to highlight a point I’m trying to make. The literature is full of transformations and research that serve as positive and negative examples of good scale-up thinking.
There are examples, however, that are less than choice in terms of green processing or good scale-up thinking. As I have said previously, green chemistry and good scale-up principles may not be equivalent concepts, but they can and often do run in parallel.
An interesting transformation is featured in the recent article entitled Efficient 1,2-Addition of Aryl and Alkenylboronic Acids to Aldehydes Catalyzed by the Palladium/Thioether-Imidazolinium Chloride System, by Kuriyama, Shimazawa, and Shirai, J. Org. Chem., 2008, 73, 1597-1600. [My apologies to the authors for their unanticipated role in this analysis.]
In this article a bond forming reaction between 1.5 eq of a boronic acid and 1.0 eq of an aldehyde is described affording a secondary alcohol. The transformation is catalyzed by 0.5 % Palladium allyl chloride dimer with 1 % of a custom imidazole carbene precursor in the presence of 2 eq CsF as base. The reaction mixture is heated to 80 C in dioxane and the chemistry is reported to be over in ca 20 minutes.
I am somewhat reluctant to be critical of chemistry that is done catalytically and is high yielding. But this transformation, solid science though it may be, would be difficult to justify taking to scale-up without an examination of alternative schemes. Let me explain my thinking.
First, on the basis of atom efficiency alone, this process requires that a lot of different elements find their way into the pot. The tally is C, H, N, O, Cl, B, Pd, Cs, F, and S to just make a C-C bond to produce a benzyl alcohol. A scale-up chemist would have to ask, why not use a Grignard and the aldehyde? Granted, there may be incompatible functional groups on either Ar1 or Ar2 that would not tolerate a Grignard reagent. However, it is worth pointing out that the conventional way of making boronic acids is by addition of a boronic ester or fluoride to RMgX or RLi followed by hydrolysis. Compatibility is an issue there as well.
One might object that many of the diverse atoms used in the reaction are at a catalytic level and as such may not constitute a major cost or environmental insult. True enough for the user of the process. But the metal complex must be manufactured somewhere at a larger scale for distribution. Pd mining and beneficiation requires energy inputs and generates wastes. The same idea applies to the imidazolinium salt.
The reaction does seem to require 1.5 equivalents of boronic acid and 2 equivalents of cesium fluoride. Boronic acids are specialty synthetic intermediates whose manufacture generates its own waste stream. Furthermore, boronic acids can be on the expensive side. The use of a boronic acid as a latent nucleophile for a straightforward addition to an aldehyde seems somewhat extravagant.
Cesium fluoride residues (2 equivalents) will find their way into the aqueous waste stream and possibly to an incinerator where the solids may end up in roadway pavement or a landfill. While fluoride is an efficient base in this case, common sense suggests that carbonate may have a more benign fate in the environment owing to the fact that it decomposes to water and CO2. Unfortunately, the best yields are with cesium as cation.
Chemists seeking to apply this kind of coupling chemistry would be well advised to be extra careful in their IP diligence. The use of metal catalyzed coupling reactions may already be patented or applications may be pending for patents. The same comment applies to the use of imidazolinium carbenes. Industrial chemists would be well advised to look deeply into the carbene species for process and composition of matter claims. Ever since the Bayh-Dole Act, university patents have been popping up like dandelions.
I do not want to be too critical of this chemistry. It is an interesting transformation and certainly may be of use for some kind of product. But for scale-up, at first pass it seems too far from earth, air, fire, and water. I would say that for maximum profit, this process is more of a Plan B or Plan C scheme.
From NIMBY to BANANA
The 2005 government report entitled Peaking of World Oil Production: Impacts, Mitigation and Risk Management, by Hirsch, Bezdek, and Wendling, is a sobering tally of the current picture of oil production and consumption in the world today. Often referred to as the Hirsch Report, the authors take a “now shot” of the global oil production scene and speak directly to the matter of mitigating the approaching economic disruption that must usher an unprepared nation into a future of peak and declining oil production.
If you read the Hirsch Report and pay attention to current events, you may be gripped by a kind of cognitve dissonance, or a haunting sense resembling a schizophrenic episode of contradictory voices in the collective consciousness. While the global warming showboat is paddling up and down the Mississippi blowing steam and calliope music, nationalized oil producers are failing to answer calls for increased production in reply to a dramatic ramp-up in petroleum demand. Some call for increased exploration and others call for drop in replacements for petroleum. All the while, evidence accumulates that the ecosystem suffering from consumption and waste generation.
As with any discussion involving economics, it is possible for people to speak imprecisely when discussing supply and demand. Econobrowser takes Hirsch to task in this manner. It seems that many of us confuse demand with desire.
Supply equals demand today, supply will equal demand in 2025, and supply will equal demand in 2050. Whatever Hirsch means by “peaking of world conventional oil production,” it certainly isn’t the condition that “production will no longer satisfy demand.”
Our news media, now almost fully morphed into a perverse mix of gibbering Bill O’Reilly clones and entertainment news programming, prattles endlessly about the hurtful gasoline prices and truncated vacation plans. Government makes flatulent noises about more drilling, but hardly a peep about reduced consumption. Where is the journalist corps? Who is asking the tough questions?
In isolation, either climate change or an exponential oil shock are more complex than nimrods leaders in the Bush administration can process. Together, these stresses add up to a major challenge to the way we live. Maybe the situation is more complex than any nation can reasonably respond to. With global prosperity comes global demand for resources. Western nations have built a house of cards based on cheap petroleum. Instead of wage growth in the past 20 years, we have been given easier access to credit. Instead of increased savings, we have found ways to burn up discretionary income.
A major part of what has to happen to adapt to the new reality of petroleum scarcity is a remodel of our infrastructure. We need more passenger rail lines and terminals with the necessary right-of-way issues taken care of. Workers need to live closer to their place of employment. The airlines have to figure out how to operate profitably with reduced passenger miles. We must upgrade our electric power distribution system to accommodate the increasing reliance on electrical energy. If wages do not change, we must adapt to having less discretionary income to spend.
But a remodel of infrastructure will require that we adapt to living nearer to it. In the past, a proposal to build a power plant is met with a chorus of outrage or “concern”. It used to be called NIMBY- Not-In-My-Back-Yard. The latest acronym is BANANA- Build-Absolutely-Nothing-Anywhere-Near-Anything. New power transmission lines and generating plants will have to go up and it will have to happen somewhere. People naturally fret about real estate prices and their view from the dining room window. I foresee more exercise of eminent domain in the future.
How to pass organic chemistry
WordPress shows the blogger what search terms lead the searcher to your blog. One of the searches that lead a reader to this blog was “How to pass organic chemistry”. Here is my answer-
Don’t get behind. Study study study study study study study study study study study study do the problems study study study study study try to enjoy it a little study study study study study study study study study study study study study study study study study study study do the problems study study study study study play racketball study study study study study study study study read the chapters 3 times study study study study study study study study study study study study study study study understand exactly what the problem asks study study study study study try to enjoy it a little study study study study study study study study study study study study study study study study study study study do the problems over again study study study study study try to enjoy it a little study study study study study study study study go back and scan an earlier chapter study study study study study study study study study study study study study study study study study study study take a few breaks study study study study study study do the problems study study study study study form a study group study study study study study study buy the solutions manual study study study study study study study study study study study study study study study study study study study study study study study study study study do the problems again study study study study study form a study group study study study study study study study study study study study study study study study study study study study do mechanisms on a blackboard study study study study study go dancing study study study study study study study study read the chapters 3 times study study study study study study study study study study study study study study study buy a model kit study study study study study try to enjoy it a little study study study study study study study study study study study study study study study study study study study find a girlfriend study study study study study try to enjoy it a little study study study study study study study study learn to draw structures using perspective study study study study study study study study study study study study study study study study study study study study study study study study study study study take a pottery class study study study study study pay attention study study study study study study study study learn the mechanisms study study study study study study study study study study study study study study study study study study study study study study study study study study do the problems study study study study study try to enjoy it a little study study study study study study study study study study study study study study study study study study study look up the structure of a medicine study study study study study try to enjoy it a little study study study study study study study study read the chapters 3 times study study study study study study study study study study study study study study study do the problems study study study study study go have tacos at 1 am study study study study study study study study study study study study study study study study study study study try to draw 3-D renderings of the structures study study study study study try to enjoy it a little study study study study study study study study read the chapters 3 times study study study study study study study study study study study study study study study study study study study take a few breaks study study study study study study do the problems study study study study study attend a study group study study study study study study learn the mechanisms study study study study study study study study study study study study study study study study study study study study study study study study study study visit the prof during office hours study study study study study try to enjoy it a little study study study study study study study study study study study study study study study study study study study go have beer & pizza study study study study study recopy your notes study study study study study study study study read the chapters 3 times study study study study study study study study study study study study study study study do the problems study study study study study try to enjoy it a little study study study study study study study study study study study study study study study study study study study go out on a date study study study study study push electrons study study study study study study study study summarize each chapter study study study study study study study study study study study study study study study study study study study study study study study study study study study do extra problems study study study study study try to enjoy it a little study study study study study study study study practice drawing structures study study study study study study study study study study study study study study. Get some sleep.
1950’s Chemistry
I recently spent some time listening to an acquaintance talk about his days as a student at MIT and as a grad student at Harvard in the early 1950’s. He had Geoff Wilkinson for inorganic chemistry at MIT as an undergrad and later did his PhD with Wilkinson at Harvard. Curiously, Wilkinson did radiochemistry in the Manhattan Project prior to joining academia. His radiochemistry experience compelled him to work fast and in test tubes, according to my friend.
My friend’s lab mate in Wilkinson’s group was Al Cotton. They started grad school together ca 1952 or so. This was shortly after the sandwich structure of ferrocene was proposed by Wilkinson’s fellow Harvard prof R. B. Woodward. Woodwards basis for this structure was on symmetry and a single IR stretch absorption. Spectroscopically, the original sigma bonding model didn’t fit the data. Just prior to this, Wilkinson had begun work on a variety of organometallic Cp compounds. As the story goes, when Woodward expressed interest in making more Cp compounds, Wilkinson went to his office and “had words” with Woodward. Afterwards, Woodward moved on to other things.
My friend laughingly recalls the time he was chewed out by his P-Chem prof, the great George Kistiakowski and earlier, by Arthur Cope at MIT. He recalls being summoned to Cope’s office. Cope was wearing pink slacks which contrasted with his red hair. He was displeased about the impertinent back channel invitation my friend pitched to Linus Pauling to speak to the chemistry club. (I haven’t verified the color of Cope’s hair)
My friend recalls having E. J. Corey as a lab assistant while in an undergraduate lab at MIT. He joked that he saw Corey once at the beginning of the term and once at the end. My PhD advisor, Al Meyers, did his post doc with Corey some years later. Small world.
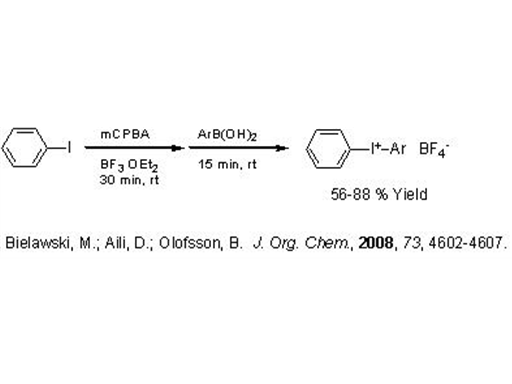
Preparation of Iodonium Tetrafluoroborates
An interesting bit of chemistry was published by Berit Olofsson at Stockholm University in a recent JOC. The Olofsson lab has previously produced a method for the one-pot preparation of diaryliodonium triflates. This latest work provides diaryliodonium tetrafluoroborates (JOC, 2008, 73, 4602-4607).
The preparation of I(III) compounds usually starts with an Ar-I compound undergoing oxidation followed by an electrophilic addition/substitution to another arene. Regioselectivity is obtained by choosing a donor with a leaving group such as a boronic acid, stannane, or silane.
What is clever about this process is the fact that a BF4 salt is directly produced. Two equivalents of boron trifluoride etherate are used in the reaction which evidently results in some kind of disproportionation producing the BF4 counter-anion.
It is known that the reactivity of iodonium compounds is somewhat sensitive to the coordinating ability of the counter-anion, so BF4 is less undesirable than other choices (like chloride). Solubility is greatly influenced by the choice of counter-anion as well. This is particularly true in photo-initiator applications where the choice of carrier fluid may be limited.
Structural diversity of organic chemistry
The recent issue of Journal of Organic Chemistry, (JOC, 2008, 73(12)) has a few articles that are particularly interesting.
The article by Lipkus, et al., entitled Structural Diversity of Organic Chemistry. A Scaffold Analysis of the CAS Registry, JOC, 2008,73, 4443-4451, is a particularly ambitious bit of work that only CAS could do. This article describes a scaffold survey of more than 24 million organic compounds in the CAS Registry.
The data set was limited to carbon-based structures containing the heteroatoms H, B, Si, N, P, As, O, S, Se, Te, and the halogens. Moreover, the work was further limited to framework structures containing rings or linked rings. Acyclic compounds were not included owing to the inapplicability of the framework definition in the search algorithm. Multicomponent substances and polymers are ignored as well.
Lipkus and coworkers found that half of the graph frameworks analyzed are described by only 143 framework shapes. The remaining half are described by 836,565 graphs.
One of the key conclusions is quoted here-
“It is not surprising that some frameworks occur much more frequently than others. However, the extreme unevenness in the way frameworks are distributed among organic compounds is somewhat surprising. This is particularly true at the graph level, where it is found that only 143 framework shapes can describe half of the compounds. The fact that both graph and hetero frameworks have very topheavy distributions tells us that the exploration of organic chemistry space has tended to concentrate on relatively small numbers of structural motifs.”
Lipkus concludes that cost minimization is one of the drivers of this “… shaping the known universe of organic chemistry.” He comes to this conclusion due to the presence of a power law which describes this distribution. The power law he refers to is a linear log-log relationship that is indicative of what they refer to as the “rich-get-richer process”.
If I understand this correctly, a relatively small number of easily made or commercially available early precursors are comprised of ring graphs that, by virtue of modification, propagate into more complex analogs that retain the original graph. This has the effect of multiplying the frequency of a given graph.
The cost minimization aspect comes from the benefits of familiar chemistry and the commercial availability of a fairly limited set of ring graphs. Adding more rings will usually mean adding more molecular weight and adding problematic synthesis and separation issues.
The authors conclude that the lopsided distribution of organic compounds toward only 143 graphs comprises a bottleneck in drug discovery. They further suggest that more exploration in other areas of chemistry space may be worthwhile.
Verbund Manufacturing
German manufacturing culture does many things very well, but a few things particularly stand out. One of these items pertains to the concept of verbund manufacturing. Verbund simply means “integrated” or “linked”. Verbund manufacturing sites are clusters of manufacturing units that take advantage of proximity. Clustering can offer certain logistic and energy advantages if done intelligently.
A cluster of manufacturing sites can operate and share a co-generation plant for the distribution of steam, waste heat, and electricity. Large capital items like steam plants can be shared so funds can be plowed into larger scale for better economy. Rail operations and other transportation resources can be shared as well. Clustering also provides for the possibility of vertically integrated manufacturing on site and a reduction in transportation costs.
Clustered manufacturing may also have the effect of concentrating the supply of skilled workers for the labor pool. A manufacturing nexus can attract community colleges and other vocational opportunities for the next generation of employees.
The USA has many manufacturing sites where similar industries congregate. Look at the Gulf coast with all of the refinery locations. But the extent to which there are synergistic interactions between companies is unclear.
In the US, corporations tend to behave as the Republic of Exxon or the Republic of the Union Pacific. This kind of a fragmented confederation of corporate states is becoming obsolete as we go up against nationalized business entities that control key resources and trade. The key to future vitality is greater efficiency with resources. Synergistic cooperation is one model that is available. But to do this requires trust and the desire to cooperate for mutual benefit. Competition begets gamesmanship and posturing which works against the verbund model for US businesses.
US corporations have much to learn from this business model.
Chemists and Engineers
What would happen to innovation in chemical technology if we had a more intimate comingling of chemistry and the engineering sciences? What effect would there be on the stream of chemists graduating into the world if more schools had a chemical engineer on the chemistry faculty? Could a single engineer on the faculty actually make a difference in altering the direction of the boat a few degrees?
Why is such a change desirable? One way to change the trend of deindustrialization and economic repositioning of manufacturing out of North America is to stimulate innovation in the industrial sciences. To do this we can rely on business leaders individually to formulate strategic plans to upgrade plants and processes by way of step changes in technology. But for business leaders, the calculation for such a change must also take into account the alternative of moving production to another country. Many times it is easier and faster to move production to China rather than taking a gamble on the invention of better technology. A large amount of pharmaceutical manufacturing has been shifted to China, Mexico, and India for this very reason.
To rely on business leaders (top down) to ramp up innovation really means that one is relying on the market. While letting the marketplace drive the economics and distribution of manufacturing has a certain appeal to purists, the global marketplace is highly distorted by government and taxation. Letting “pure” market forces govern innovation as the sole driver is to bet all of your money on a horse that limps. Why not find ways to stimulate innovation with an improved stream of chemical innovators and a renewed urgency?
Universities do this all of the time. But it is my sense that other disciplines perhaps do this better. It is all too easy for we chemists to invent a reaction or composition, publish it, and then move on to the next outcropping of opportunity. We do this thinking that surely somebody will pick up the ball and run it to the end zone of commerce.
But for any given paper published in SynLett or JOC or ______, the likelihood of commercialization is low. It is not automatically the role of academic science to drive its work towards commercialization. That has been the role of engineering.
What has been lacking is more significant early overlap of the two disciplines. For a chemist to truly be a part of bringing a transformation to the manufacturing scale, the chemist has to begin thinking about how to prepare the chemistry for the big pots and pans. This is what the art of scale-up is about. And in scale-up, the practice of chemistry has to overlap with the practice of engineering.
Industry already provides for itself in this way by training chemists to do scale-up work. This kind of work has always been beyond the scope of academic training. But what if there were a course of study wherein chemistry faculty and students could more thoroughly address the problems of chemical manufacture? What if engineering concepts would be allowed to creep into the training of chemists?
Chemistry faculty would begin writing grants for process oriented research. Schools without engineering departments might start hiring the odd engineer or two in an effort to “modernize” the chemistry department. Gradually, a department might become known among recruiters and donors for producing a strain of BS, MS, and PhD chemists who are already adapted to process research.
It is important to stress that the goal is not to plop conventional engineering curriculum into the chemical course of study. That will not work. But what is possible is to build a minor in industrial chemistry applications. This pill will be easier to swallow for the P-chemists because in short order it would be apparent that chemical engineering is heavily loaded with physical chemistry.
I have tried to make a case that one way to make a positive influence in chemical innovation in North America is to begin a grass-roots effort to stimulate the culture of chemistry. I believe that providing an avenue of study that includes early exposure to engineering and process economics will stimulate many more students and faculty to make significant contributions to entrepreneurism and industry.
Blog TV
Wow. It has finally happened. Bloggers now have a medium for split-screen video streaming in Blogging heads.tv.
Good heavens. I don’t think I am ready for this. I’ll have to get a bow tie …