Summary: The point of this essay is to remind people that, while the works of Charles Darwin and Jean-Baptiste Lamarck were obviously profound in the understanding of many aspects of the biology of life on earth and its adaptation to the environment, their work is very much a product of the mid-19th century. This was prior to the atomic and molecular theories of matter were developed. Since that time the fields of biochemistry and molecular genetics have grown to a high level of sophistication and provided many mechanistic details on how evolution can occur at the level of molecules. With the advancement of biochemistry and molecular genetics, evolution is recognized as a molecular phenomenon using chemical mechanisms not unfamiliar to chemists. It seems likely that if Darwin, Lamarck and others did not make their early contributions to evolutionary theory, biochemists and biologists of the 20th century would certainly have proposed evolution as an inevitable consequence of the mutability of life.
………………..
The frame of reference in this essay is that of an organic chemist’s mechanistic view of the fundamentals of chemical change in biochemistry or molecular biology. Let’s just call it chemistry.
Of the many features of popular science content, one annoyance to the writer stands out: Articles on evolution remain fixated on Charles Darwin’s mid-19th century opus magnum, “On the Origin of the Species“. Darwin’s survey expeditions on the Beagle from 1831 to 1836 as a gentleman companion and naturalist resulted in sharp observations, sample collection, notes, books and years of scholarly lectures.
The question of biological change from evolution dredged up considerable controversy early on, most prominently from the religious communities and lasting to this very day. Much later, after chemistry based on atomic theory was well established, creationists began to sermonize on the statistical problems with the right atoms coming together is the correct order to produce a person. The mantra was that creation implies Creator. Within the context of the Abrahamic religions, the creation of life was clearly stated in religious texts. To assert otherwise was simple heresy. Eventually, the more literate opponents of evolution latched onto the physical principle of entropy.
Entropy is a concept that creationist’s love to unsheathe and swing around. They will say that the 2nd Law of Thermodynamics opens up an apparent contradiction. The crux of their argument is based on equating entropy with ”disorder’. Life itself is comprised of many kinds of highly ordered matter, but the universe is supposed to be getting more disordered. How can this be?
What doesn’t get mentioned is the considerable disorder produced from the life and growth of organisms.
/*Begin Editorial Comment*/
The term “disorder” is the Disneyland word for entropy. It is a highly over-simplified cartoon word meant to describe entropy, which is a thermodynamic state variable. Entropy has the physical units of energy per degree Kelvin per mole. It refers to irreversibly dispersed energy in a system. In my opinion, the word “disorder” is too loosey goosey a definition for even a loose definition.
/*End Editorial Comment*/
A protein molecule doesn’t appear to be “ordered” to the untrained eye. However, a protein molecule has 3 levels of structure in its final construction. First is the specific sequence of amino acids in the protein chain. Second, this protein chain consists of chemical bonds that are free to rotate and chemical bonds that are not as with the peptide bond. This rotational freedom of motion allows a protein chain to rotate about many of its chemical bonds and come to rest in a place where two features may have a reversable mutual attraction. Or, the protein relaxes in a particular configuration that has the least strain.
A third form of protein structure comes from the attraction of individual proteins with another. Proteins often align with another to from large complexes, frequently imbedded in cell walls. These protein structures can contain a channel where ions may pass from interior to exterior of the cell. The channel can be opened or closed in response to external stimulation.
As a protein chain is assembled, it has amino acid features in it that can form hydrogen bonds which allow particular stretches of the chain to reach around and weakly and reversibly connect with itself. An amino acid that has a thiol (or sulfhydryl, -S-H) group can react with another to form a disulfide linkage (-S-S-). The disulfide linkage is a covalent linkage and thus somewhat stable though is subject to reductive or oxidative cleavage.
A length of protein can form a helical secondary structure, a somewhat flattened secondary structure called a beta-pleated sheet, or an unstructured sequence of amino acids.
A few words about entropy, S
One of the ideas frequently cited in creationism is entropy. It is cited because they take evolution as contrary to entropy and the Second Law of Thermodynamics. According to Google, the thermodynamic definition of entropy is given as-
Unavailable Energy: In thermodynamics, entropy quantifies the portion of a system’s thermal energy that cannot be converted into useful work. The more disordered a system, the less energy is available for work.
A more expanded definition is-
Entropy is fundamentally linked to the second law of thermodynamics, which states that the total entropy of an isolated system always tends to increase over time. This means that systems naturally move towards a state of greater disorder and less available energy for work.
The usual interpretation is that the total entropy of the universe is always increasing. This gets interpreted as the world becoming more ‘disorderly’. Unfortunately, the word ‘disorderliness’ can be cognitive bias. The natural meter-scale world we reside in provides many examples of orderliness, which is often just a value judgement by people seeking tidiness.
Creationists often portray abiogenesis and evolutionary change as highly improbable, suggesting the ultra-minuscule chance that the necessary atoms could connect perfectly to create life. If this process was truly random, I might concur. However, the formation of molecules from atoms and the subsequent reactions leading to further changes are not entirely random. Any two atoms or molecules colliding are subject to random motions, true enough. However, what happens during and after a collision and subsequent reaction is far from random. At Earth surface temperatures allowing liquid water, atoms and molecules engage in water compatible reactions, yielding a limited array of possible outcomes and sometimes even a sole outcome. Given certain conditions such as temperature and chemical surroundings, each atom or molecule is restricted to a fairly small number of reaction channels or pathways. Life did not spontaneously arise or evolve from a purely haphazard broth of atoms.
Careless assertions about entropy and the order/disorder of matter can lead to specious conclusions.
The better definition of entropy comes from statistical mechanics. Entropy describes how energy is distributed among the microscopic states of a system. It describes how many ways the system can be arranged at the microscopic level while still appearing the same macroscopically. [From ChatGPT]
Entropy as disorder is perhaps a cul-de-sac rather than the road to understanding.
The Creationist’s respect for the 2nd Law is quaint, but it is more a matter of picking up their opponent’s club and beating them with it in error. When finished, they set it back down and walk away satisfied that they have used science to beat science.
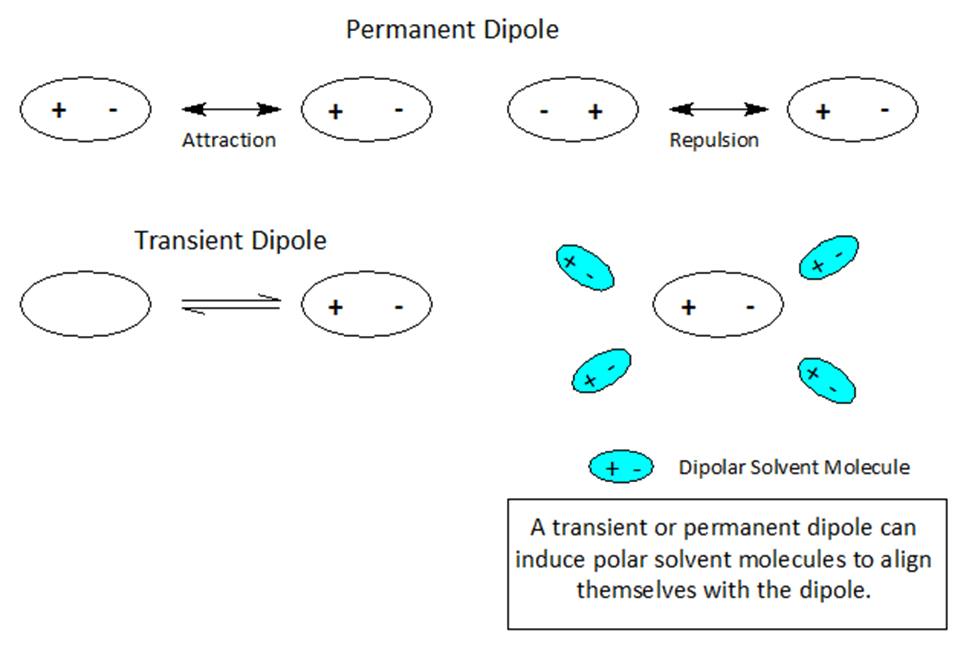
Dipoles- Nature’s Sticky Spots
At the atomic level of matter during a reaction, atoms or molecules may undergo a rearrangement of charge leading to +/- ionic species producing a single pole or a dipole.
When atoms and molecules undergo electronic change the surrounding solvent environment may help or hinder a given transformation. If during the course of a chemical reaction a transient charge is produced, the solvent ‘bag’ enclosing the reacting molecules can promote or hinder the reaction transformation.
My personal policy is to limit the word ‘entropy’ to subjects related to the atomic scale or to heat engines. A loose pile of bricks should rather be described as in disarray.
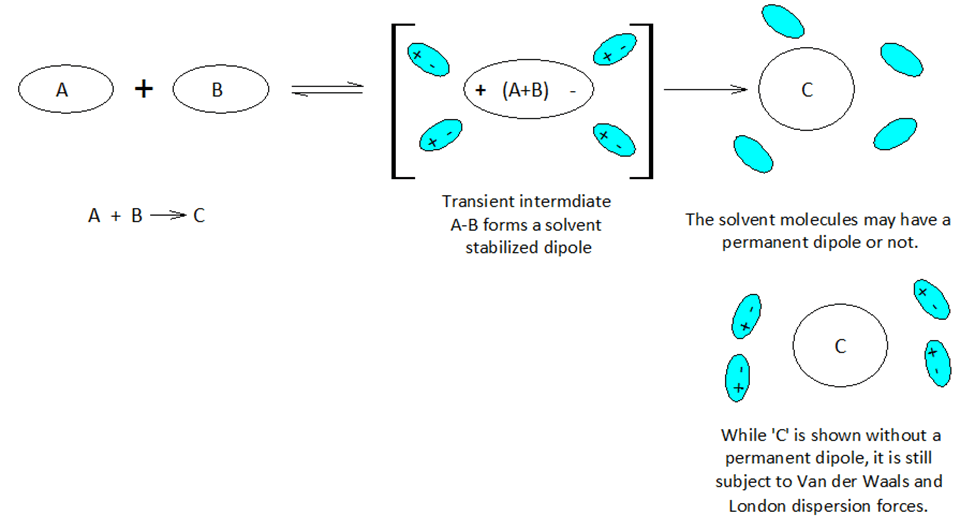
Molecules and even neutral noble gas atoms can form transient dipoles, causing small, short lived attractive forces between atoms. These are called Van der Waals forces. Graphics by Arnold Ziffel. Graphics by Arnold Ziffel. The ease with which a reaction mechanism proceeds may be subject to solvation effects. Formation of a dipole requires that negative charge is pulled away from positive charge. This takes the application of work against the natural attractive force between positive and negative charges. It takes energy to electronically alter a molecule to produce charge separation to form a dipole. A shell of dipolar solvent molecules around a reacting dipolar molecule can stabilize accumulating polarity in a molecule sufficient to aid the transformation.
Chemical reactions proceed mechanistically in a stepwise manner, and with over 150 years of extensive and peer reviewed chemical research and development, much chemistry has become quite predictable across a wide range of substances. A crucial aspect of modern organic chemistry is the understanding of reaction mechanisms. Biochemists focus on the mechanisms of reaction in aqueous environments, while classical organic chemists commonly avoid water in lab work. However, the principles of physical chemistry support both fields. Indeed, physical chemistry is the cornerstone of the chemical sciences.
On mutation
My greatest hangup about language commonly used to describe evolution is when someone says “The _______ evolved the ability to _______ in order to survive.” True, but In the minds of many this may suggest that a specific genetic change was purposely triggered to achieve the ‘goal’ of enhanced survivability. If a genetic change occurred that improves survivability, it begins randomly. It’s rightly been said that evolution is blind going forward. The DNA of an organism struggling for survival will not automatically give rise to an offspring that have resistance to a given threat. Rather, with each successive daughter cell there is a chance that a beneficial mutation has occurred. But mutations could happen anywhere in the genome. Some mutations may be beneficial, and others may be problematic in terms of survival. There is a chance that the mutation may never be expressed from dormant genes. In order for a mutation to pass forward, it must happen before or during reproduction. Mutations to the parent organism after it has reproduced end right there, beneficial or not.
Radiation-induced mutations to the DNA are more likely to occur during mitosis in cell division when copied DNA strands are being pulled apart and into the daughter cell. The DNA strands are not yet wound with the histones and are more accessible to external influences like radiation or chemical insult.
/* Anecdote: DNA Breakage */
In my second go-around with radiation for prostate cancer in spring of 2024, I learned that the practice now is to deliver approximately the same overall radiation dose as before, but in fewer and larger doses. The idea is to cause breakage in both DNA strands of the double helix rather than just a single strand in the cancer cells. I had 4 sessions of 8 gray in 2024 as opposed to 21 sessions of ~1.5 gray in 2014. I cannot account for why the total dosages are not equal, but there were specific cancerous tissues like the prostate and the seminal vesicles to hit the first time around.
/* End Anecdote */
Today there is a growing understanding that there are actions with the DNA polymer-histone structure that do not involve changes in the genetic sequences. This is called Epigenetics. The total human DNA double helix stretched out is approximately 2 meters in length yet must be contained within a cell. The way that DNA double helix does this is to wrap around a series of individual proteins called histones for compaction into a smaller structure called chromatin. Finally, the chromatin folds into the familiar chromosome structure.
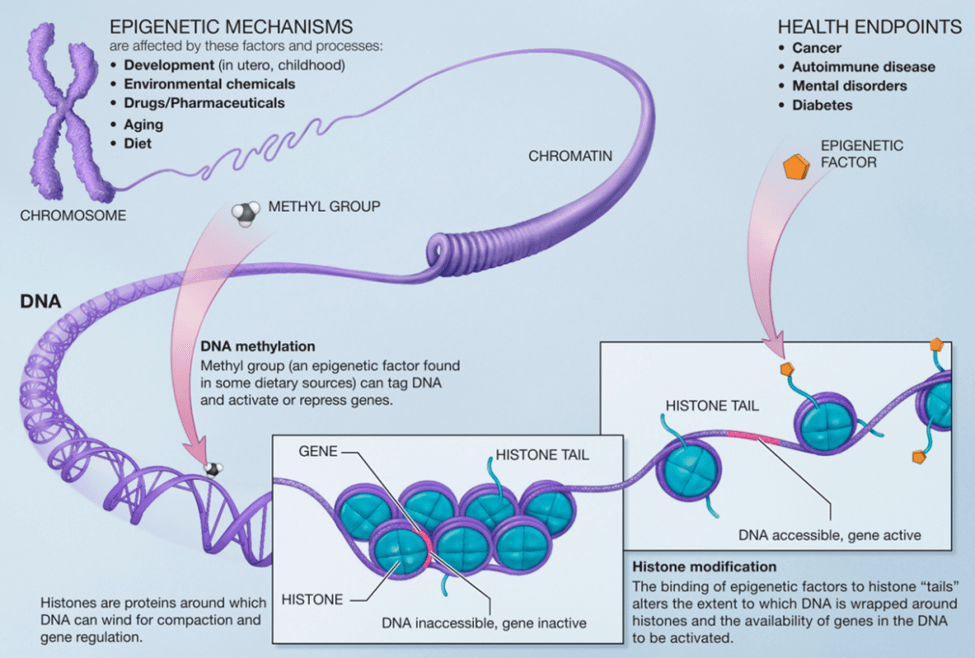
But is that adaptation by the existing genome composition? Genetic evolution is blind going forward. If a species evolves with a survival advantage of some kind, there can be no “foresight” involved. If it is truly genetic evolution, the end result is because of heritable genetic changes at the level of molecules. If a changing environment causes altered expression of an existing gene in response, say a gene that is otherwise dormant but is suddenly “awakened” by the new environment somehow, then perhaps this is a form of “adaptation within the existing genome” rather than evolution by editing of the genome. This is where epigenetics operates.
Charlie Darwin
The naturalist, geologist and biologist Charles Darwin‘s claim to fame is substantial and well deserved. His book “On the Origin of the Species” is the work that is cited by many but read by few. Over his lifetime he had published considerable work before Origin of the Species. What may be less known is that the notion of evolutionary change wasn’t something that he alone scraped together. Others had previously speculated out loud and in print about changes in species over time. His grandfather, the physician Erasmus Darwin, produced a volume titled Zoonomia that anticipated some of the work of Lamarck which foreshadowed the concept of evolution.

Charles Darwin drawing by Samuel Laurence, 1853. Source: Wikipedia.
In 1831, Charles Darwin embarked on what was originally planned as a two-year voyage of discovery aboard the H.M.S. Beagle, but which ultimately spanned five years. The expedition’s primary goal was exploration, and Darwin, recommended for his scientific interests, joined as a gentleman naturalist with Captain Robert Fitzroy, rather than as a mere specimen collector. Fitzroy, a Vice-Admiral in the Royal Navy and a scientist, led the journey. Darwin, during the voyage, dispatched bones, fossils, seeds, illustrations, and writings back to England, garnering significant interest from geological and natural history circles. Of interest, prior to setting sail Darwin had acquired skills in taxidermy.
After Darwin’s return to England, he spent many years speaking, writing, rewriting and publishing his accounts of the voyage. He is buried in London’s Westminster Abbey just a few meters from the grave of Sir Isaac Newton.
Our acknowledgement of the value of Charles Darwin’s work and methodology is fully legitimate. Darwin’s theory of evolution was a major step change in how we think about biology, speciation and introduced us to natural selection. What is missing from Darwin’s work, however, is the physicochemical mechanism of how evolution works. This is understandable simply because biochemistry was unknown at that time. Inheritance at the molecular level was a mystery until the early-mid 20th century when the molecular biology of the gene began to come together. DNA and RNA had to be isolated and characterized as well as observations made of their x-ray structures. The connection of DNA and RNA polymers to protein formation and composition had to be arduously worked out. Accounts of this are easily found on the internet.
One point of this essay is to argue that while Darwin and others began to coalesce the varied observations of macroscale adaptation and speciation found around the world into a grand theory, the mechanism of evolution lay at the Ångstrom to nanometer scale of the molecule. I find it impossible to deny that if Darwin and others had not come up with evolution at the macroscale, biochemists would have discovered molecular evolution and it would have been used as a basis for the evolution of species.
Sidebar. Rosalind Franklin (25 July, 1920 to 16 April, 1958)
Much acrimony has been made over the alleged snubbing of physical chemist Rosalind Franklin in the selection of 1962 Nobel Prize winners in Physiology or Medicine for the discovery of the structure of DNA. A recent paper in the 25 April, 2023, issue of Nature brings together some little-known details of the cold-shoulder given Franklin as a co-discoverer of the double helix structure of DNA.
The disqualifying event for the 1962 Nobel Prize occurred in 1953 with Franklin’s death. At the time, posthumous awards were accepted only if the death occurred between nomination and the award date which was not the case for Franklin.

Dr. Rosalind Franklin. Source: Wikipedia.
The story of the discovery of the double helix structure of DNA involves 4 central characters: James Watson and Francis Crick from the University of Cambridge, UK; Rosalind Franklin and Maurice Wilkins at King’s College, London.
Before any of this started, the involvement of deoxyribonucleic acids in heredity had been suggested by Oswald Avery in 1944 (below). Watson and Crick, Franklin and Wilkins did not discover DNA. They did, however, use x-ray diffraction of crystalline DNA fibers and model building to deduce the chemical structure of B DNA.
At King’s College at the time of this story, the biophysics group was led by John Randall whose deputy was the New Zealand-born biophysicist Maurice Wilkins. In 1951 Franklin joined the Department on a 3-year fellowship having come from Paris where she used x-ray diffraction to study the structure of coal. By this time Wilkins had been working on DNA since 1948. A personality clash arose between the more assertive Franklin and the less confrontational Wilkins, so Randall divided certain DNA samples between them. Franklin received a calf’s thymus-derived sample of the highly purified material from the Swiss chemist Rudolf Signer – Wikipedia at the University of Bern and Wilkins received a “poorer sample” from Chargaff at Columbia University in NYC.
Sidebar to the sidebar.
Chargaff had become interested in DNA after Oswald Avery at the Rockefeller Institute published a paper in 1944 concluding “The evidence presented supports the belief that a nucleic acid of the desoxyribose type is the fundamental unit of the transforming principle of Pneumococcus Type III“. Avery and colleagues had developed the first immune serum for a strain of pneumococcus from the blood of horses. Along with colleague Michael Heidelberger they found that polysaccharides associated with this strain of pneumococcus could be isolated from water-soluble spherical capsules around the cocci and are antigens, which later led Heidelberger to discover that antibodies are proteins. These are now fundamental facts in molecular biology and immunology.
Back to the double helix
Wilkins had earlier discovered that there were 2 forms of the DNA in solution- the crystalline A form and the paracrystalline B form. Franklin took the A form and Wilkins the B form. Franklin discovered that the A form will convert to the B form in higher humidity and revert back to the A form in lower humidity.
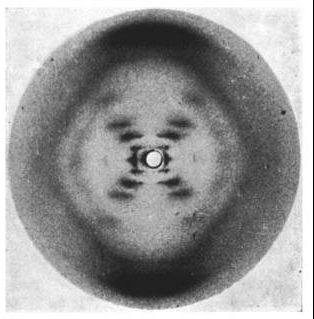
Photo 51. The x-ray diffraction pattern of the “B” form of DNA, taken by Raymond Gosling while working under Wilkins. Source: Wikipedia
Unfortunately for Franklin with the A form, Wilkins’ B form is what is found in the cell.
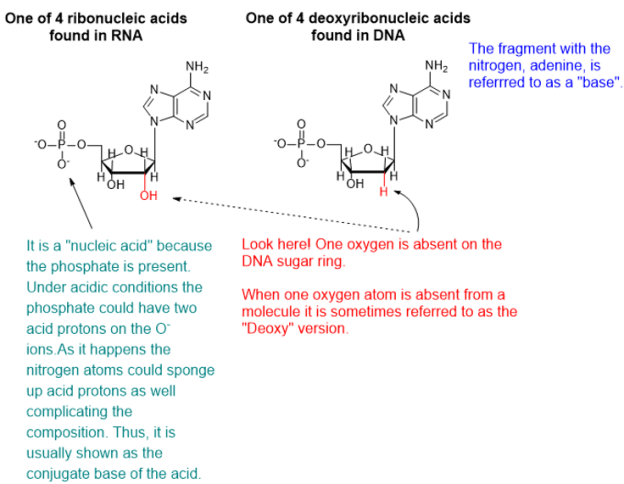
From a biochemical standpoint, mutation of DNA sequences makes chemical sense and today is routinely observed in DNA assays. An increasing number of diseases or characteristics are linked to distinct mutations in DNA. Deoxyribonucleic (or desoxyribonucleic) acid (DNA) is a chemical substance that, like all chemicals, is susceptible to its chemical environment and whatever particular substances happen to be nearby or to ultraviolet or ionizing radiation or to highly reactive chemical species like free radicals.
Biochemical Evolution
Charles Darwin is renowned for his well-articulated, evidence-based argument on the evolution of species by natural selection. He courageously introduced a detailed new theory within the conservative British scientific community. His ideas fascinated many leading naturalists of the time, who adopted and furthered the theory. Truly, it marked a considerable progression for that period.
“But but but, it’s just a theory!” This is a common objection by creationists and religious zealots thinking they have found the weak underbelly of evolution. They claim it is “just” a theory as though a theory was merely a fanciful excursion of the imagination where all opinions are of equal validity.
A theory is an overarching explanation or model subject to improvement over time with which arguments are made in support of or against a core concept. This core concept is initially built on a pedestal of clay. As better analysis and experimental data come in, the pedestal is strengthened or weakened. Furthermore, the theory may be unequally affected across its breadth with some aspects perhaps tossed out and others supported. Theories themselves evolve and strengthen with evidence. Scientists are naturally anxious to contribute to sorting out the truth of a theory.
Another objection to evolution is the previously stated notion that “creation implies the existence of a creator.” A common argument is that a watch is such an unlikely collection of highly refined components that there must be a watchmaker behind it. The human hand or eye are the anatomical examples often cited. There is a bit of vocabulary that muddles these arguments. The use of the word “creation” presupposes that the universe is something that was assembled by a creator. If you see the world as something that had to have been created, then the idea of evolution may be difficult to swallow.
The question of life on Earth has two important aspects to it. One is the evolution or change that species undergo over time. The other is the initiation or abiogenesis of life from non-living matter. Of the two areas, the evolution is the most developed concept.
Biochemists and molecular biologists have taken Darwin’s evolution from a mid-19th century macroscopic theory supported by the fossil record, geological observations and the gross anatomy of animal species from around the world to the submicroscopic machinations of molecules. This has been a gigantic leap forward in understanding not just in the chemistry of all life but also the evolutionary physicochemical mechanisms of life. Life is one of the things that chemicals can do given opportunity and time.
Christian and other churches reacted negatively to evolution in Darwin’s time as most do today. Darwin and many geologists concluded that the Earth was far, far older than did scholars withing the church. So, what is this about? Are church leaders skeptical or just stubborn? Is this even a good question?
Types of thinking
I would offer that people can be spread between two bookends in regard to thinking: Devotional thinkers and analytical thinkers. Devotional thinkers have a core doctrine supporting their beliefs and think and behave in a way that their belief guides them. Devotional thinkers study their doctrines in an effort to be in better alignment with it. It is not uncommon for devotional thinkers to limit their exposure to things not aligned with their devotion. Devotional thinkers are sometimes labeled faithful. Their goal is to study supernatural doctrine and align one’s personal behavior.
Analytical thinkers will naturally adopt a baseline worldview that comports with their education, observations and logical sensibilities. But when presented with new data or just a compelling idea, analytical thinkers may be persuaded to open new vistas in their thinking or at least set the idea aside as new thinking under consideration. Analytical thinkers are sometimes labeled as skeptics.
It is impractical to approach each new circumstance one encounters ab initio. In order to explain how an airplane flies it is not presently necessary to first independently derive Newton’s laws of gravity and Prandtl’s fluid dynamics so as to set the stage for lift and drag forces. Everyone has a practical baseline picture of the world that serves as a conceptual starting point for some kind of conclusions on reality. The discipline of science is highly vertical with old knowledge built upon or revised by new knowledge. The requirement for accuracy is practiced by the investigator and checked upon by peer reviewers. Nobody wants to be that scientist who has published a paper with faulty science requiring a retraction in the Retracta Acta.
To be skeptical of the evolution of the species is on one hand to require supporting evidence and compelling arguments. On the other hand, many people dismiss evolution altogether as being contrary to their faith-based notions while posturing as an “evolution skeptic.” However, when physical evidence or collected data are thrown on the table for all to examine and when that evidence is part of a trail of evidence logically or mechanistically interconnected, then to dismiss the logic or measurements is to go beyond skepticism. Apologists would claim that they are keeping their faith against adverse influence or even resisting evil. But standing against evidence could really be considered simple stubbornness for fear of perceived divine consequences or discomfort.
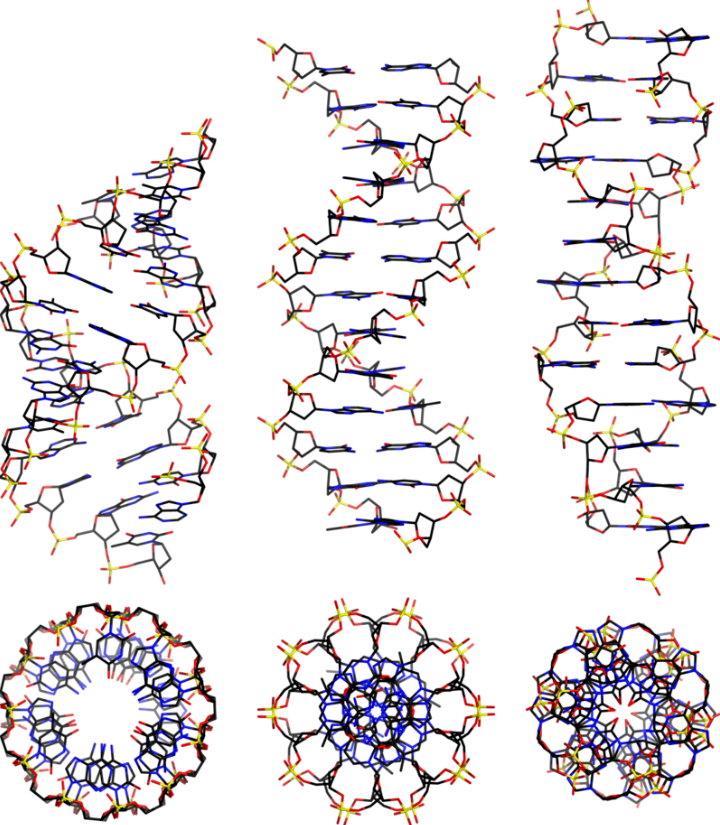
DNA and RNA are polymeric substances comprised of four major subunits. Three of the subunits are shared by both DNA and RNA and the fourth is a different component characteristic to RNA. This determination took some time to arrive at the correct structures and the chemical mechanisms.
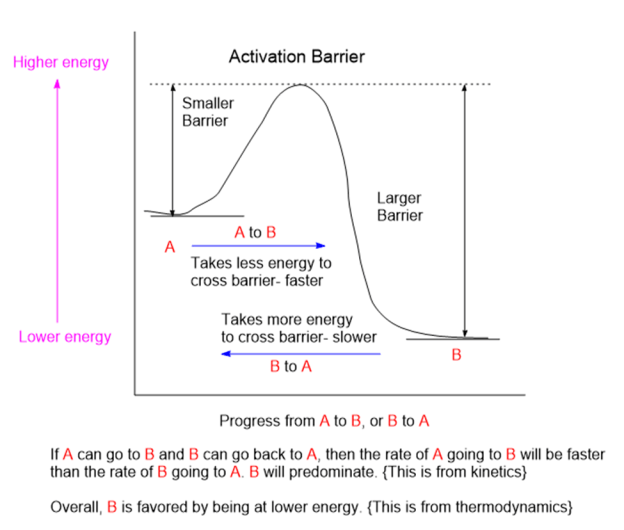
Chemicals interact by particular mechanisms depending on what is present and physical conditions like temperature, pressure or interfering substances. These mechanisms are a built-in, reliable feature of matter in our universe. When multiple mechanisms are possible, the fastest one tends to prevail, channeling matter down that pathway. The fastest channel will have to lowest energy barrier to cross. However, if the reverse mechanism is possible then a balance will be struck between two reservoirs of substances. This is the basis for thermodynamic equilibrium. The reaction direction with the lower energy barrier will be faster, and if the reverse direction isn’t possible, the mechanism will preferentially populate the direction with the lower energy barrier. We would say that the reaction is under kinetic control. If the reaction can go both ways, then a balance will be struck producing substances on both sides of the energy barrier producing thermodynamic control.
One argument offered by Creationists is that the probability of all the atoms in a human coming together to form that human is 1 in 10stupid large. In other words, they say, highly improbable within the age of the universe. And if that was how it works, then I’d agree. But it is definitely not how chemistry and evolution work.
Evolution happens by a biochemical ensemble of mechanisms in solvent water constrained by the boundaries of chemistry and physics and specifically to what is possible in aqueous media. Liquid water is necessary rather than solid or crystalline phase water because for bio- or any chemistry to operate, molecules have to diffuse around and collide in order to react. Biochemistry and therefore evolution occurs at temperatures between roughly -10 oC and 45 oC, plus or minus a bit and at midrange pH levels.
Life is substantially based on carbon because carbon forms stable chemical bonds with nitrogen, oxygen, sulfur, hydrogen and especially with itself. Phosphorus appears as phosphate. Carbon can form chains of indefinite length and 3, 4, 5, 6, 7 and 8-membered rings or larger with 5 and 6 being the most common ring sizes. Tens of millions of different chemicals structures are possible within this group of elements. Nature is crammed with ring systems in natural products.
Biochemistry is not based on silicon, even though silicon has certain chemical similarities to carbon. Silicon does not easily form chains greater than 2 silicon atoms in length and it has a strong affinity to oxygen. This affinity is very much thermodynamic in nature and is difficult to overcome chemically at biological temperatures and pH. Silicon-nitrogen bonds are hydrolytically unstable at low to moderate pH. All of this adds up to poor utility for silicon in biomolecules.
Each of these carbon-nitrogen, carbon-oxygen, carbon-phosphate, carbon-sulfur, carbon-hydrogen and carbon-carbon bond combinations as well as the various combinations of N, O, P, S, H atoms have their own variations as well. Other atoms like iron, calcium, sodium, potassium, magnesium, chromium, selenium, iodine, and a few others serve purposes other than for molecular skeletons generally.
The point of citing all of these combinations of atoms is to emphasize that each has unique chemical properties and unique reactivities. The slapdash Creationist assertion that evolution merely brings atoms together to form an organism and no consideration of reactivity is mentioned. While molecules in solution are more or less randomly banging around, their entry points for successful chemical interactions are far from completely random. In fact, a given molecule will react only in a few ways depending on what it collides with. A complex molecule like glucose has several reactive sites, but it still has a limited menu of reaction types available at physiological conditions.
Molecules are tiny objects that can have even smaller features where changes can happen. These features can only do certain kinds of chemical reactions. They are called ‘functional groups’ and they are limited to a limited set of reaction mechanisms, often only one mechanism.
Think of each of these limited types of reactions as a channel. Overall, biochemical transformations happen through these particular channels. There may be numerous channels possible on a molecule affording diverse reactive outcomes. Even among the possible transformations, a few channels will react faster and thus dominate. The point is that there are not an infinite number of ways that molecules can exhibit reactivity. This means that evolutionary change through biochemical mechanisms does not have an infinite set of likely chemical pathways. There can be many, to be sure. But the entire ensemble of biochemical mechanisms operating in an organism do not have to change to allow a given evolutionary change.
Evolutionary changes can occur in very subtle ways. A biochemical modification may result from a misreading of the normal genetic code or from some other off-normal situation, but this is not an evolutionary change. A genetic change can result from an alteration in the sequence of the genetic code itself. A change in the sequence of the DNA may lead to a heritable mutation if the change is survivable. A mutation in an unused stretch of DNA may occur and lead to no effect. If the genetic change is fatal to the new cell, cell death can occur, and the mutation will not be passed forward.
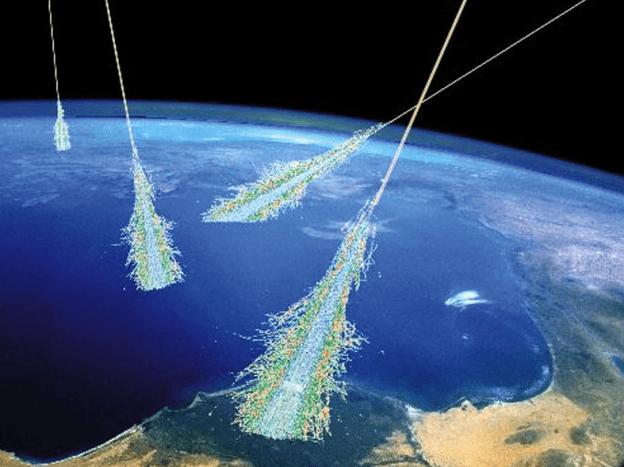
Energetic cosmic radiation from outer space and the sun is constantly showering the Earth’s upper atmosphere. When a particle or a photon from space impacts a person, it will penetrate to some depth, dumping kinetic energy into tissues that can break chemical bonds to form ion pairs or radical species. Ion pairs can reconnect as before or with other species to form new substances. Radicals are neutral atoms or molecules where electrons in the form of lone pairs or covalent bonds are evenly split into 2 radical species where each is neutral but have unfilled octets. Radicals tend quench themselves by popping off a hydrogen radical from a nearby molecule or by colliding with the radical that was originally separated.
Radiation exposure of living tissue or other material objects produces ‘stochastic’ damage because the kinetic energy of the radiation particle or photon far exceeds the energy needed to cause bond breakage or general scrambling of biomolecules. Stochastic radiation damage is fairly unselective so, as the radiation passes through materials, the energetic particle dumps some or all of its energy into the material directly along its path of movement.
One measure of the potency of a given particle or photon of radiation is the number of ion pairs produced per inch or centimeter. The three major types of radiation are alpha, beta and gamma. Alpha particles produce the most ion pairs because of its high kinetic energy so it dumps its kinetic energy along a very short distance. Beta particles can travel a bit longer distance (several inches) but require less shielding than gamma rays. While gamma rays are quite penetrating, their ion pair production is very low.
Any given human exposure to radiation can result in no observable effect or tissue damage. Not every exposure to radiation will result in cancer. Because radiation damage to tissues is stochastic, a survivable mutation of DNA is random.
Enzymes are proteins that act as catalysts or enablers of chemical transformations. These protein enzymes have places on them that are clefts, ridges and valleys along their exterior where other molecules or coenzymes can collide and interact called an active site. It is possible for the enzyme to be active continuously and catalyze transformations when the right substrate molecule jostles along. It is also possible for the enzyme to require ‘activation’ in order to function. One means of activation comes from phosphorylation of the substrate to be acted upon, phosphorylation of the enzyme itself, while yet another is where an external molecule binds to a particular spot, resulting in an alteration in the overall shape of the enzyme. This alteration in the enzyme’s shape can open the active site of the enzyme and allow the intimate contact necessary for a particular molecule to diffuse in, bind and undergo a catalyzed transformation. In this way, an enzyme can be deactivated as well.
Much new drug discovery and design is based on toggling an enzyme “off or on” with a suitable substrate. The substrate can be constructed so as to be highly specific, to be detachable or to sacrifice itself by covalently connecting to the enzyme and prevent it from further functioning. This last category is sometimes referred to as a suicide substrate inhibitor. Penicillin is an example of a suicide inhibitor that covalently combines with an enzyme, shutting it down permanently. Penicillin and its many analogs have a strained 4-membered ring in them called a beta lactam ring that can relieve the strain by ring opening to a straight chain by connecting to a feature on the enzyme. This is irreversible though bacteria have developed the ability of reacting with penicillin to eliminate its ability deactivate a target enzyme.
Enzymes can be very sensitive to a change of one amino acid which could lead to little change or it could cause the enzyme to operate a few percent faster or slower. Or it could change the reaction rate or specificity by a great deal. It might even allow different substrates to be acted upon by the enzyme. Let’s say that this change in the operating rate of the mutated enzyme causes a chain of successive biochemical reactions to operate faster, say by increasing the efficiency in the use of energy. If this results in the survival rate of the organism increasing by a bit, it may impart a survival advantage. If the alteration of the enzyme reduces the survival rate, then the organism may continue to survive or not.
But hold on. Evolution is blind going forward. Not all changes register as an advantage or even show an effect. If the mutation happens after reproduction, then it does not get inherited by succeeding generations. If the mutation is fatal, then the cell dies and the genetic change halts. If the process of evolution is so iffy, how does anything happen?
We should reflect on how fast chemical reactions can happen. At room temperature, water is undergoing collisions at a rate of ~1010 per second. Now imagine 1 mole of water, 18 grams, all molecules undergoing ~1010 collisions per second. One mole of water contains 6.02 x 1023 water molecules. Simple mindedly, that adds up to 6.02 x 1033 collisions per second in those 18 grams, or 1 thirsty swig of water. But that’s not all. These water molecules are also vibrating, rotating and translating at perhaps 1012 vibrations per second. In general, each collision will carry a particular probability of a bond breaking or bond forming event. Water is a boring example, but we can see that a reactive biomolecule is also undergoing a very large number of collisions per second, each with a certain chance of participating in a reaction. Even though a given reaction may be of low probability per collision, a great many collisions raise the odds of fruitful interaction.
Rapid molecular collisions in combination with a limited range of reaction channels means that the molecules will sort themselves out by way of finding the lowest energy-barrier, fastest reaction channel to follow. This is far from completely random. The fastest reaction channel can consume its inputs the fastest and the product from this fast channel will predominate.
Why would there be DNA, protein or other biomolecules that are fragile enough to suffer mutation in the first place? Why hasn’t DNA evolved into a sturdier structure free of mistranslation, mutation and other errors in its functions? There are certainly substances that are more robust than DNA or RNA like hydrocarbon polymers, silicates, urethanes, urea linkers and other polymers that are much more stable to chemical insult. The DNA double helix is, after all, held together by low energy hydrogen bonds.
A key requirement of life as we know it is that something has to prompt DNA to unravel and split strands of deoxyribonucleic acid chains apart. In order to unravel, the structure holding it safely in the double helix form must be capable of assembling and coming apart when prompted. The nucleic acid structure along each chain of the double helix have phosphate linkages. Because phosphoric acid is a weak mineral acid it can lose one, two or three acid protons (mono- di- or tribasic) under physiological conditions.
The phosphate linkage in DNA works very well for life. It allows free rotation about the linkage and is quite polar for good compatibility with water. Phosphate can form bonds between themselves: 1, 2 or3 phosphates can link, leading to short chains of phosphate anhydrides. Because phosphate is relatively stable under physiological conditions yet is able to function, it is nearly ideal for its purpose. Phosphate is phosphorus (V) with 4 oxygens bonded in a tetrahedral fashion. Three of the oxygen atoms have single P-O bonds with one P=O double bond. When connected as anhydrides, mono-, di- and triphosphate anhydrides may form. The linking oxygen atom connecting the two end phosphates can be displaced and added to another substrate. This is called phosphorylation and is critical in biochemistry.
Abiogenesis
Abiogenesis is the big puzzle at this point in history. Evolution is not the same as abiogenesis. How did life begin? As we look around at the Earth today, we see an overprinting of billions of years of planetary, geologic, oceanic, and atmospheric transformations. The present world at the surface is nowhere near that world at the time when life began to flicker into existence. One of the primary differences is the chemistry in play. Before oxygen began to accumulate in the atmosphere, many elements may have been exposed at a reduced oxidation state, that is to say electron rich. The chemistry of an atom greatly depends on the state of its valence electrons. So much so that the atom loses its identity when charged or in molecular form. For instance, +H (protium or hydrogen cation) is chemically different from –H (hydride ion) is different from 0H (atomic hydrogen)and all are different from molecular H2. Referring to +H as hydrogen is incorrect. It is properly referred to as “hydrogen ion” or “protium ion”. The ions are distinct chemical species. This range of possibilities in the state of reactive atoms (other than the noble gases) somewhat complicates the chemistry of prebiotic Earth.
At this point I’ll refer the reader to the InterWebs for deeper insight into abiogenesis.