In the US, it’s common to enhance one’s home with greenery, notably a grass lawn. However, a lawn requires ongoing attention. I have a lawn care service fertilize and treat our lawn with herbicides throughout the growing season. Recently, I’ve scrutinized the herbicides they use. They used prodiamine and dicamba.
Hold on a minute. Wasn’t the 2020 registration of dicamba nullified recently in federal court? Yes, it was. Why has it been sprayed on my lawn? The ruling applies to the use of dicamba on soybean and cotton crops that have been genetically modified to be resistant to it. The high volatility of dicamba has been quite deleterious to crops in adjacent farmland and to plants that are pollinated by bees because of drift resulting in crop losses and sharp decrease in honey production. Dicamba is a broadleaf herbicide, though not effective against grasses.
A Brief Meteorological Interlude
Nature continually directs hostility towards our lawns and gardens, both from above and the sides. Living in a semi-arid climate with only 14 inches of annual moisture, the lack of precipitation is immediately detrimental. The desiccating rays from the sun, located only 8 light minutes away, evaporate vital moisture from plants and soil. Compounding the problem, dehydrating winds whisk away the moisture cooking off the soil. Since moist air is more buoyant than dry air, it rises and is carried away by convection into the prevailing winds.
At higher elevations, the combination of increased moisture and decreased temperature can lead to cloud formation. Moisture ascending from the ground combines with the air above. The lower temperatures at these heights cause the moisture to transition from a gaseous to a liquid state, resulting in clouds. This change, although it appears innocent, has thermal consequences. For humidity to condense into liquid, the surrounding air temperature, which reveals “sensible” heat, must be low enough to absorb the “insensible” or latent heat released during condensation without causing a significant rise in temperature. If not, an increase in temperature would hasten the shift from condensation back to evaporation. There is a delicate equilibrium in this phase transition.
As latent heat is released, the air’s density decreases, enhancing its buoyancy and causing it to rise further. The ascending misty air cools, allowing more moisture to condense, which adds to the cloud’s mass. But wait, there’s more—
A rising air parcel causes the surrounding air to be drawn inward from below towards the ascending convective column. Consequently, a significant volume of air may be uplifted, enhancing the moisture levels above the ground contributing to the formation of a convective cumulus cloud. Latent heat supplies part of the energy needed for the vertical ascent of air. This cycle persists until a net downward movement of rain occurs, pulling down cooler air from higher altitudes. The cessation of upward momentum in cloud formation leads to a rapid downward surge of air with the rain, which, upon reaching the ground, spreads out horizontally, occasionally at high speeds. This explains why cool gusts of wind often signal the approach of a rainstorm.
Prodiamine
On to Prodiamine and Dicamba. These two herbicides provide broad coverage by virtue of different biochemical mechanisms. Dicamba is a selective postemergent broadleaf systemic herbicide.
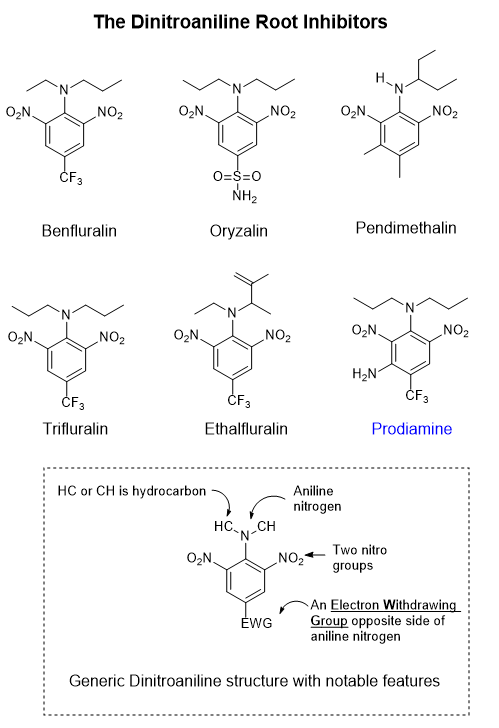
Prodiamine is a pre-emergent herbicide effective on crabgrass and annual blue grass, goosegrass, spurge, chickweed. A pre-emergent herbicide like prodiamine is injected into the soil where it binds to soil particles. A close analog called Trifluralin, prodiamine without the NH2 group, has been shown to have sufficient volatility that sufficient vapor can penetrate root tissue where it expresses its activity.

Prodiamine was invented by Sandoz and went to market in 1987. In 1996 Sandoz merged with Ciba-Geigy to form Novartis. In 2000 Novartis spun out its agrochemicals and genetically modified crops business and with AstraZeneca formed Syngenta, which is now part of the Syngenta Group, purchased by Sinochem in 2017.
Comments on Patenting of Chemicals
When a biologically active substance is discovered, usually is the case that particular features and the shape of the molecule are crucial to the activity. Not just attachments but also the spatial relationship between them. The subject molecule is likely to be active in interacting with a pocket on an enzyme. That pocket has a particular 3-D shape that the molecule has to fit. Not only that, but the enzyme pocket is likely to have protein amino acid groups that have an affinity for charged or water insoluble features on the incoming molecule.
Looking at the prodiamine structure and analogs above, we can see that all of the analogs share certain features: two Nitro groups, -NO2; 1 Aniline nitrogen group with one or two hydrocarbons attached, -N(hydrocarbon)2 groups; a single 6-member hexagon ring (a benzene ring) from which to hang all of the appendages. Opposite to the top aniline nitrogen is an attachment present which 4 of the 6 analogs have: a -CF3 (trifluoromethyl) group attached. This doesn’t happen by accident- someone decided that it should be there because something useful happens with it there. A -CF3 group acts to pull electrons in the ring to lean in that direction, affecting how the electron charge is distributed on the whole molecule. Another analog has a -S(=O)2-NH2 group. This thing, called a sulfonamide group, also pulls ring electrons towards it. Why -CF3 versus -S(=O)2-NH2? Perhaps one is more potent or selective than the other or possibly because one was claimed in a patent and at the time the other was not. Either one could be a me-too herbicide. Analogs of a basic motif arise frequently in a competitive marketplace.
Often times, when a new and successful motif of pharmaceutical or agrochemical comes along, the race begins for competitors to develop close analogs, though being careful not to infringe on any patents. With chemical patents the composition of matter can be claimed, the method for making the substance as well as the method of use. Composition of matter, method of manufacture and use claims are often split into separate patents for IP safety in case one patent gets knocked down. What’s more, a composition of matter patent can be written so as to claim a vast number of analogs to broaden the IP real estate. This is called a Markush claim where a variable letter substitutes for a large or small set of chemical groups. A single structural framework can have many Markush groups giving rise to an astronomically large set of claimed combinations. Some companies, hide the composition of the best analogs in the Markush claims so as to minimize competitive intelligence losses to competitors.
Dicamba
A weed is a valueless plant growing wild that is in competition with a desired crop. The three major morphological categories are: grasses, sedges, and broadleaf weeds. A weed represents lost soil fertility.
Dicamba is a member of the benzoic acid subgroup of the aromatic carboxylic acid group of herbicides. This group of compounds are synthetic auxins, or plant hormones, that interfere with plant growth.

Other popular herbicides
Other carboxylic acid herbicides besides dicamba are the 2,4-D analogs.
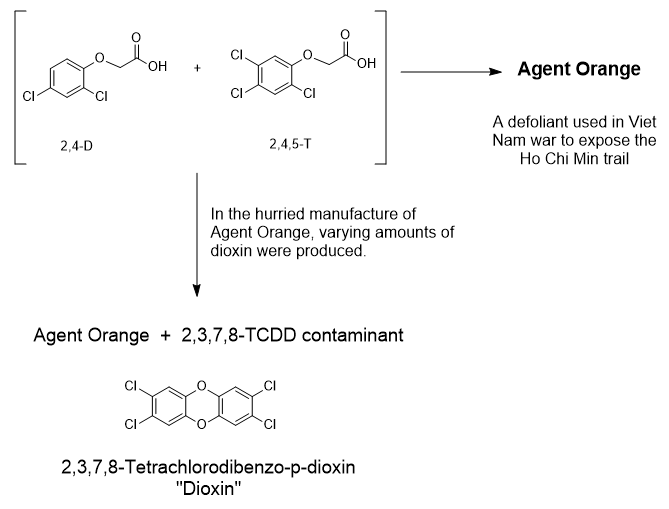
Of the numerous forms of the dioxins, the species that is often discussed is the 2,3,7,8-TCDD version. The positions and number of chlorine atoms varies. The mechanism above shows the dioxin analog coming from 2,4,5-T. The 3-ring structure of TCDD is the dioxin core structure.
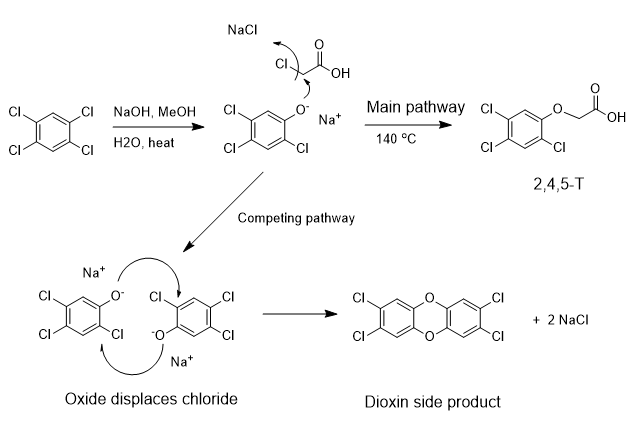
2,4-D is a synthetic auxin, similar to dicamba in mechanism, that causes uncontrolled and unsustainable cell growth. The herbicide is absorbed through the leaves and is moved to the meristem where uncontrolled cell growth follows.