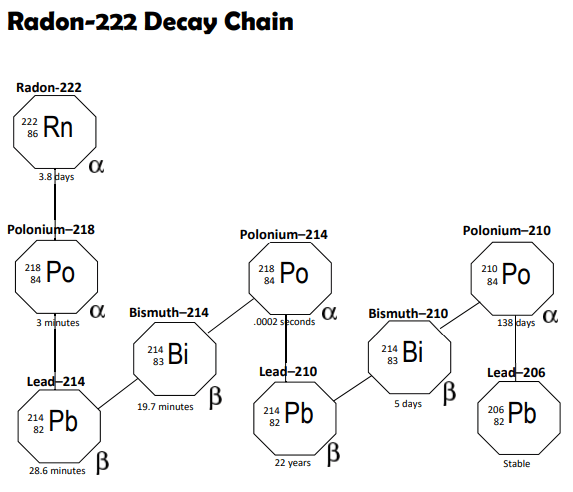
Being a dopamine addict with a persistent Fear of Missing Out (FOMO), I spend far too much time scrolling on social media. Today I ran into a thread about the shelf-life of American distilled liquors in Canada. It seems that the Canucks find themselves heavy with American distilled spirits after the decision to stop their sales in Canada.
The question was about the shelf life of distilled spirits, particularly Kentucky Whiskeys, etc. There is much hand wringing by Canadian liquor store owners in the frozen north. What are they to do with expired liquors which they have or will have in abundance?
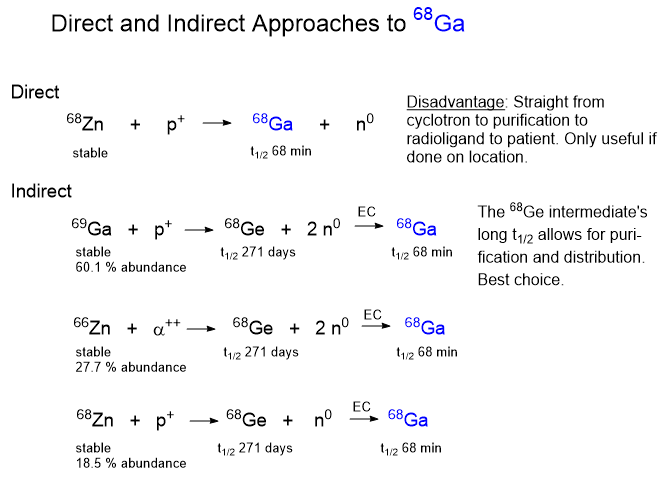
Whiskey (or equivalently “Whisky”) is substantially comprised of ethanol and water. Flavor components include esters, aldehydes, ketones, phenols, and other organic molecules. Distilled and concentrated ethanol, such as Everclear, is diluted to the desired proof and then stored in wooded barrels. The much-praised aging process is really about extracting flavor chemicals from the charred wood of the barrel.
Of copper and sulfur
The aggrandized folksy-woodsy wisdom of the distillers of whiskey is a trifle exaggerated. The use of copper stills is often trotted out to convince the public there is closely guarded secret knowledge in the production of whiskey. According to this link, the use of copper in distillation equipment early was largely due to the availability and ease of shaping and forming copper in the fabrication of distillation equipment. Additionally, copper has truly excellent heat conductivity which aids in both heating and cooling. Our friend, Mr Google, says that copper absorbs the sulfur fermentation components from the fermented mash. Well, copper and sulfur do have mutual attraction as evidenced in copper minerals and ores. It is reasonable to expect some amount of contamination of copper surfaces by sulfur containing components. On basic principles, with exposure to heat, air and sulfur, the copper surfaces can also be expected to be passivated eventually, reducing effectiveness in sulfur removal. To date I’ve heard nothing about this.
Back to regularly scheduled programming
The industry I spent most of my career was specialty chemicals. We used organic solvents exclusively. The word “organic” just means carbon-based. The question of shelf-life was rarely a riddle we needed to noodle through because of rapid turnover. We did take storage precautions with ethereal solvents because of the well-known peroxide hazard.
Some bacteria are aerobic, requiring oxygen to survive. Others may be strict anaerobes, growing only in the absence of oxygen. Others may be facultative meaning that they can grow with or without oxygen. Bacteria can be in a vegetative state meaning that they ae actively growing and reproducing cells. Some bacteria are spore forming meaning that they can enter a dormant state, reactivating when environmental conditions are right. In general, vegetative bacteria are active and spores are inactive. Vegetative bacteria can be killed with heat, chemicals or radiation. Spores are partially dehydrated and resistant to heat, chemicals or radiation.
Both the vegetative and spore forms of bacteria can reside in liquids, surfaces, on biological tissues or in suspended droplets in the air. Freshly pasteurized milk can be contaminated with bacteria only to start the growth cycle again. Such contamination can come from packaging equipment, empty packaging containers, or by sloppy handling by workers. In our plant, milk packaging was sanitized with a quick spray of hydrogen peroxide prior to filling. But hydrogen peroxide isn’t a universally potent sterilization food grade chemical. We once had bacterial contamination getting into our fluid milk after packaging. It puzzled everyone but me. At the time I was enrolled in undergraduate microbiology and was familiar with Pseudomonas aeruginosa. This organism forms blue colored colonies and released a fruity, not unpleasant odor.
In the dairy lab, we routinely plated all products in agar petri dishes at 15 process minute intervals and incubated them. Suddenly that day we were finding green colored plates, but with the sweet P. aeruginosa odor. Then I realized that blue bacteria mixed in with yellow agar would appear green. I borrowed some specialized growth media from the university for clinching the identity and we nailed it: P. aeruginosa was only weakly pathogenic, limited to the sick and the elderly. We disposed of the contaminated stretch of the production run and sanitized very aggressively. This solved the problem. Better living through science. However, I quickly received a vigorous tongue lashing from the plant manager for using advice and specific growth media from my micro professor. Still, we solved the problem, haha.
Shelf-life or product expiration depends entirely on the particulars of any given substance. For instance, ethers in general may have a shorter shelf-life than hydrocarbons due to organic peroxide formation. This is especially true if the container has been opened and air exposure has occurred. Remember that peroxide quenching additives like BHT are stoichiometric in their function and may be consumed to exhaustion over time by air infiltration.
There is expiration due to microbial growth including bacteria, yeasts and mold, as well as chemical degradation of substances. Then there is expiration due to liability containment where a producer won’t guarantee quality indefinitely. Instead, the producer may use a tried-and-true in-house approach or SOP defining some arbitrarily chosen, rounded number period like 3 or 6 months, 1 year or longer. The food industry settles on freshness dates based on knowledge of their product aging data. Over time a food item may naturally become inedible or unattractive in appearance for several reasons. This is easier to measure.
Dairy product expiration
For instance, in the dairy processing plant all products had a labeled expiration date. Milk should be safe to drink up to 1-week past code date, but only if it hasn’t been allowed to warm to room temp (rt) by sitting out. The shelf-life dropped to about 1 day if it did warm to rt. Regular homogenized milk (homo) requiring refrigeration is not sterile. The ordinary short time, high temperature pasteurization process does not aim for zero viable bacteria. Milk labeled Ultra Pasteurized is sterile. Our rule of thumb at the time was that once the bacteria loading went past 2000 bacteria per milliliter of milk, an off flavor can be detected and the milk should be discarded, at least for matters of flavor. Bacterial infection and illness from milk is scarce today since pasteurization came into use.
Off flavored milk due to fermentation isn’t necessarily unsafe to consume. After all, look at yogurt and cheese. Both are accepted microbial fermentation products. Next time you see a picture of a dairy cow, have a close look at the location of the udders. They are hanging between the hind legs and close to the soil, next to the “glide path and splat” of falling manure. Cows kept in a barn or pen are forever walking in their own manure. This simple fact alone should justify pasteurization. But today, there is some movement to consuming non-pasteurized milk product. History has proven this to be unwise.
What I learned in the dairy lab analyzing milk products for quality control of products like skim, 1, 2, and 4 % homogenized fluid milk, sour cream, cottage cheese, juices, and novelty dairy-fat items was that making cheese is no great mystery. Cheese is inevitable. Let milk sit around at room temp and it will eventually turn into cheese and whey from naturally occurring microorganisms in the neighborhood. However, it is unlikely to taste good.
Chemical-based expiration
The aging of products by purely chemical transformations is quite different from microbial aging. Microbial aging involves uncontrolled, but self-limiting, growth rates of the microorganisms which follow an s-curve. The s-curve shows that microbial aging begins slowly but soon enters the “log phase” characterized by exponential population growth. The log phase doesn’t last forever, though. Soon population growth levels off as nutrients are consumed and general growth conditions deteriorate. The loss of further nutrients leads to eventual death of the colony.
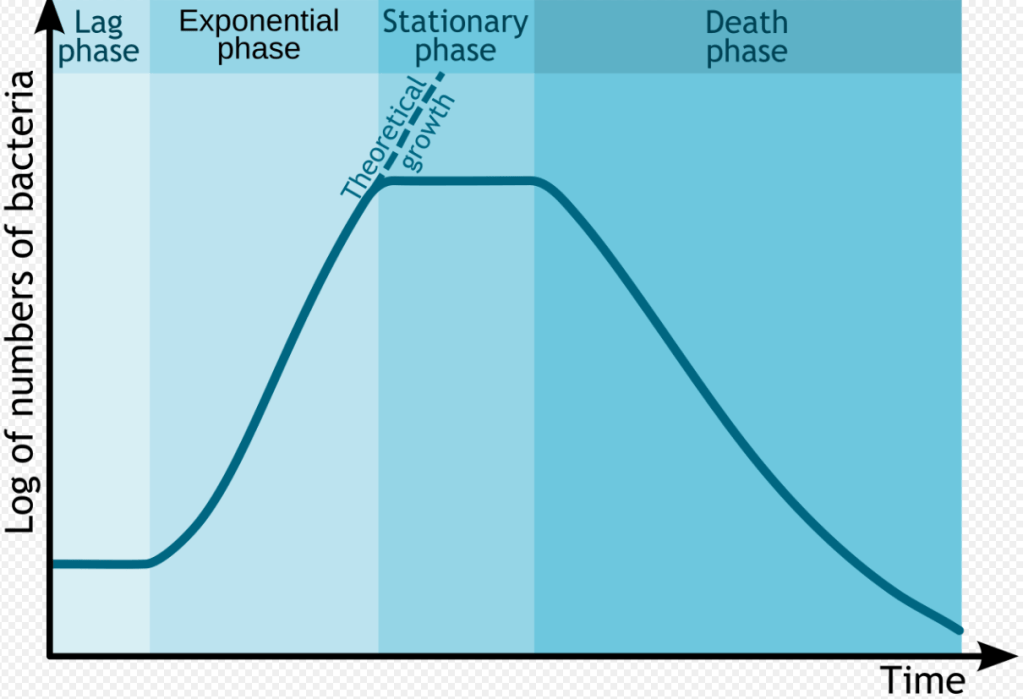
Chemical aging is a kinetic phenomenon depending on initial concentrations, temperature, time, and a rate constant This means in principle that the chemical composition of the material can be predicted, extrapolated or interpolated mathematically. Microbial growth is a population change following a logarithmic growth curve leading to a population plateau. Here, the growth rate and the death rate become equivalent. Theoretical growth might approach an asymptote, given sufficient nutrients, removal of deleterious waste products and room to grow.
An asymptotic decay or growth curve means that the slope of the curve over time never flattens exactly to zero– only closer and closer –approaching some limit. It only approaches a maximum or minimum depending on the data being observed. Sometimes the loss of the product is followed by either the product concentration itself or by following the appearance of its decay product. Here, the sensitivity of the measuring equipment may come into play with detection limits. To deal with asymptotic decay or growth, a time constant (tau) is determined. Tau is useful in several contexts including radioactive decay and chemical change or decomposition.
In generalized chemical product decay, decomposition rates will depend on the decay mechanism. Is the decomposition dependent only on the initial concentration and the decay rate of a single component. This is first-order decay with dependence on the concentration of a single component, In regard to the shelf-life of a product, a single component product decays without interaction with other components. This kind of product quality loss would produce an asymptotic decay curve, approaching but never reaching some limit.
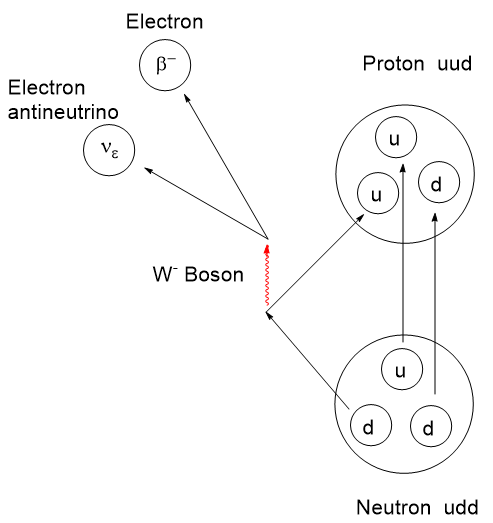
Whereas first-order decay depends on external factors like temperature and other product components, the zeroth-order decay of a radioactive source is independent of influences external to the nucleus. Radioactive decay produces beautiful decay curves.
Time constants.
When a substance or product (like a capacitor) produces an exponential decay curve, the line between acceptable quality and unacceptable must be drawn according to the business externality of specifications. In determining the shelf-life by chemical (or biochemical) decay/decomposition, because of the exponential nature of the decay process, the time constant of the property causing decay can be used to decide how long it takes to decompose down to some minimum acceptable level.
The short answer about time constants is the time it takes the quality indicator measurement to fall by 37%. I say quality indicator because the first-order decay of something may not actually be measurable easily and cheaply. The decaying component may have a large influence in some bulk property that determines the overall quality and marketability of the product. Good quality control requires the use of measurable and reliable indicators.
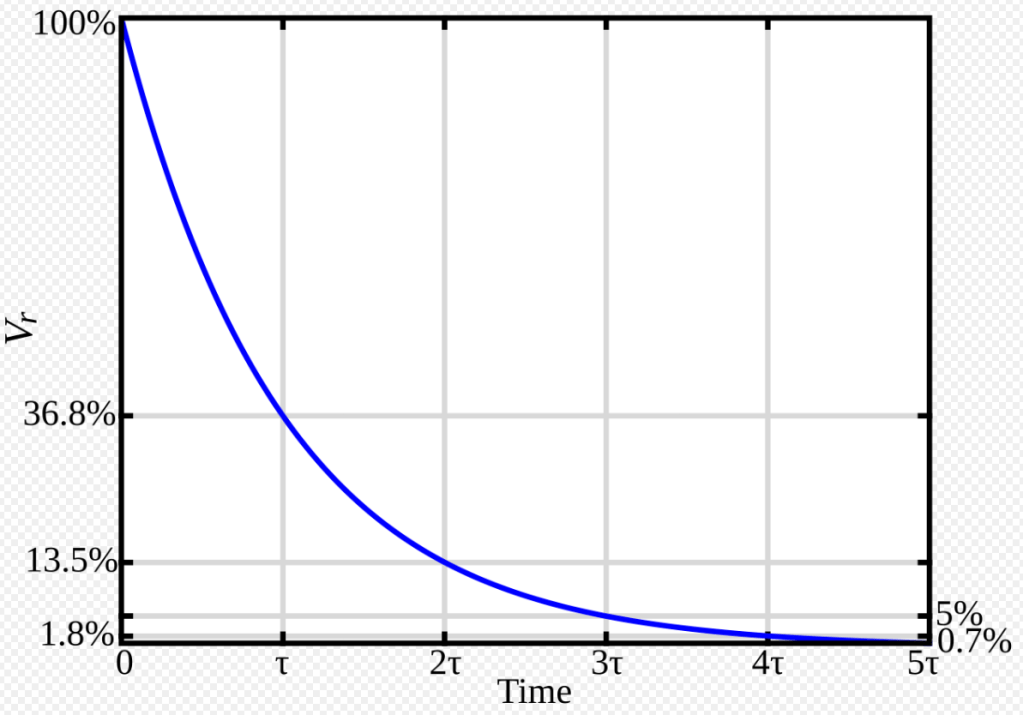
For example, if the time constant of a material is 100 days, the remaining ~63 % after 100 days will decay by 37% in another 100 days and so on until you’ve gotten to some acceptable % levels, where acceptable in the business sense means above the level needed to drop out of specifications.
The decay of an isolated electrostatic charge follows an exponential curve according to a time constant. How many time constant periods must one wait for the static charge to decay to a safe level or near zero? The answer I’ve read for electrostatic charge is 5-time constants, tau τ. As can be seen below, 5τ time periods takes you down to within 0.7 % of the theoretical result.
A Google search of a safe number of tau periods gave the numbers below.
- 1τ: Reaches ~63.2% of final value (or drops to ~37%).
- 2τ: Reaches ~86.5% of final value (or drops to ~13.5%).
- 3τ: Reaches ~95% of final value (or drops to ~5%).
- 4τ: Reaches ~98% of final value (or drops to ~2%).
- 5τ: Reaches ~99.3% of final value (or drops to ~1%).
General Use: 5τ (99% settled) is a common benchmark for “good enough”.
High Precision (ADCs, etc.): 8τ or more ensures minimal error.
Specific Events: If you just need a quick response, 1-2τ might suffice.
A Google or other search on the topic of “time constants” will provide a proper mathematical justification of this value.
Determining shelf life with time constants
Following the percentages, barring other factors, a product shelf life of xτ where x is the number taking you to minimum acceptable decay/decomposition. Added on top of this may be company policy or requirements of the customers. If only a single τ takes the product below specs, then a producer may have to rethink the value of x or make better product.
/end/