A short drive from my office is the Fort St Vrain power plant. The present electrical generating facility is powered by natural gas. But a generation ago it was a nuclear plant powered by a high temperature gas cooled reactor (HTGR). What’s more, the reactor used fissile uranium with fertile thorium. The output of the plant was ca 330 MW electric and it operated from 1976 to 1989.
The utility eventually decommissioned the helium cooled reactor and converted to natural gas. Today, as before, the plant looks like a planetary humidifier, billowing great clouds of steam condensate into the thin dessicated air of the high plains. The link above outlines the trials and tribulations the utility experienced with some of the auxillary hardware. They had to learn the principle of KISS the hard way.
A thorium-based nuclear reactor uses a fissile element like U-235 to provide a source of neutron flux from which to jumpstart in-situ breeding of U-233. The absorption of a neutron by Th-232 gives Th-233 which beta decays to Pa-233 which decays again to U-233. Remember, beta decay causes the atomic number to increase by one, but the atomic weight stays the same. The resulting U-233 is fissile and serves as a fuel.
Thorium as a fuel has pluses and minuses. On the plus side, thorium is more abundant than uranium. And Th-232, the predominant isotope, is the desired fertile material. This is in contrast to natural uranium which offers less than 1 % abundance of fissile isotope U-235. A large part of our nuclear infrastructure involves separation of this isotope to a more concentrated form. After isotopic separation the uranium must then be converted to a suitable chemical form.
The refractory nature of thorium oxide reportedly makes fuel element manufacture somewhat problematic. Interestingly, it is the refractory nature of thorium oxide that makes it valuable for use in thoria lantern mantles. The high melting point of thoria allows a gossamer web of glowing thoria (and ceria) to sit in place in the lantern burner and radiate bright white light.
On the minus side, there is no established fuel supply infrastructure to provide thorium oxide to industry. In fact, there is virtually no thorium trade in the United States today, with the latest annual US sales volume amounting to a paultry $350,000 according to the USGS. Some of the nuclear chemistry is of the thorium cycle is problematic as well.
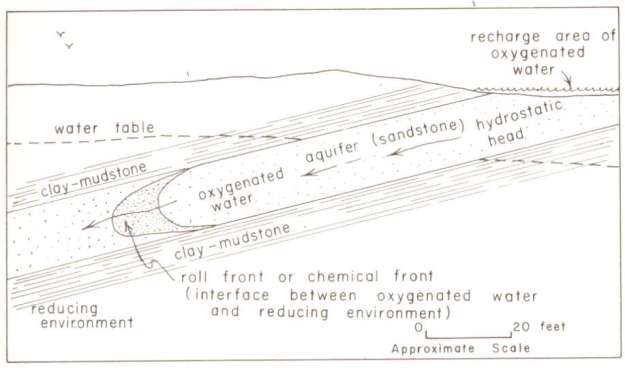
The natural history of thorium mineral placement is rather different than that of uranium. Uranium migrates fairly readily, depending on its oxidation state and pH of mobilized hydrothermal fluids. As a result, uranium can be found in porous or fractured formations that have a history of water migration. From what I can tell in the geological literature, thorium concentration results largely from magmatic differentiation in the distant past. There is considerable diversity in the details of each occurrence of thorium, so one should be careful of generalizations.
There is a notable monazite (a common thorium mineral) placer district across the central North and South Carolina border region. These monazite placer deposits sit in ancient stream channels and are the result of alluvial dispersion.
Colorado has two notable thorium mineral deposits. The Wet Mountains SW of Canon City and the Powderhorn district near Gunnison have substantial deposits of thorium as well as lanthanide elements. In fact, rare earths are commonly associated in monazite. Monazite is a phosphate mineral with a variety of thorium and lanthanide cations present. It is useful to recall that the rare earth elements include Sc, Y, the lanthanides, and the actinides. In Colorado, the significant uranium deposits are not coincident with thorium deposits. Uranium is found in sedimentary deposits of the Colorado Plateau, in the tuffaceous sediments of the Thirty-Nine Mile volcanic field, and in vein lodes along parts of the Colorado mineral belt.
There is considerable variability in the elemental associations found in rare earth deposits. Monazite seems to be fairly consistant in regard to the presence of Th and lanthanides. Scandium, however, is often absent or quite scarce in monazites from the assays I have seen in the literature.
Perhaps the richest thorium district in the lower 48 states is in the Lemhi Pass district along the lower Idaho-Montana border. A company called Thorium Energy reportedly holds substantial claims of thorium rich deposits at Lemhi.