The Environmental Protection Agency announced on August 6th, 2024 there would be an emergency suspension on all registrations of the preemergent herbicide chlorthal-dimethyl, or dimethyl tetrachloroterephthalate (DCPA or Dacthal). It has been 40 years since the US EPA has issued such an emergency suspension of registrations. This order has immediate effect.
US patent US2923634A was granted to Diamond Shamrock 1960-02-02 with a single claim-
1. THE METHOD OF CONTROLLING UNDESIRABLE PLANT GROWTH WHICH COMPRISES CONTACTING SAID PLANT GROWTH WITH AN ACTIVE AMOUNT OF DIMETHYL 2,3,5,6-TETRAHALOTEREPHTHALATE.
An early patent claiming the use of DCPA, but not the composition.
The octanol/water partition coefficient, log KOW, sometimes called Log P, is a measure of how a substance will partition itself between 2 phases, a hydrophilic phase and lipophilic phase. This logarithm is used to give some insight into the type of living tissues a substance will tend to accumulate in on exposure or dosing. A log KOH of 4.40 represents a ratio (antilog) of 25,119 to 1 favoring the octanol. This indicates considerable lipophilicity.
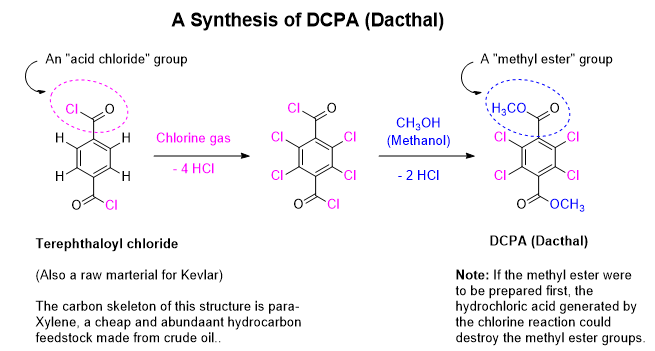
The industrial manufacture of DCPA is neither complicated nor difficult. The terephthaloyl chloride (pronounced: terra THAL oh ill chloride, soft TH as in “thing”) raw material is used in the manufacture of Kevlar and is readily made in several ways. Whether or not the DCPA manufacturer makes their own or outsources it is not available information. In either case, the terephthaloyl chloride is chlorinated to exhaustion (fully chlorinated) and then the methyl ester is prepared by contacting the chloride with methanol to form the diester (pronounced: DYE ester).
Why does DCPA have 4 chlorine atoms on it? Hard to say exactly what the thinking was, but from the process chemistry perspective forcing 4 chlorine atoms on the ring rather than just 1, 2, or 3 solves the problem of ending up with a dog’s lunch of mono-, di-, tri- and tetrachlorinated compounds in the product mix. Individually, each may have differing potency, selectivity, biochemical mechanisms, and human or environmental toxicological properties. Subsequent environmental and tox studies would be complicated by the potential of 4 analogs each possibly requiring individual testing at some point. Another thing to consider is that single component solids are much more easily purified by crystallization than a solution of solid components. A solution of mixed components can be quickly precipitated by cooling or concentrating, but pulling out one pure solid among many solid close analogs can be difficult and low yielding. Single component products are almost always better for ease of processing.
DCPA is a selective non-systemic, or contact, herbicide used for pre-emergence control of annual grasses and some annual broad-leaved weeds. Coverage rates of 6-14 kg/hectare are common.
From PubChem: “/IT IS/ PRESENTLY APPROVED FOR USE ON TURF, ORNAMENTALS, STRAWBERRIES, AND AGRONOMIC CROPS INCLUDING COTTON, SOYBEANS, AND FIELD BEANS. /IT IS/ EFFECTIVE AGAINST SMOOTH & HAIRY CRABGRASS, WITCHGRASS, GREEN & YELLOW FOXTAILS, FALL PANICUM & OTHER ANNUAL GRASSES. /IT IS/ ALSO USEFUL AGAINST CERTAIN BROAD-LEAVED WEEDS SUCH AS CARPET WEED…PURSLANE & COMMON CHICKWEED. /IT IS/ TOLERATED BY MANY CROP PLANTS.”
DCPA is a relatively simple small molecule that is made from cheap and abundant early feedstocks like para-Xylene, Chlorine and Methanol. It has good potency and desirable selectivity in its ability to kill crabgrass in the presence of turf grass. The chemical process steps are well understood, each with a long history of successful use. It can be sold in solid form or in liquid form and may be applied by a large variety of methods. It can be applied for pre-emergence or folial use.
According to EPA “DCPA is a chlorinated benzoic acid herbicide which inhibits cell division of root tips in target plants. It controls many annual grasses and broadleaf weeds in a variety of agricultural crops and ornamental varieties (e.g., broccoli, onions, tomatoes, cabbage, cauliflower, dogwood, azalea). Annual agricultural use from 1998 through 2008 averaged approximately 500,000 pounds over 100,000 acres with broccoli and onions accounting for 79 percent of that use (Ratnayake, 2011). Information also suggests that on average 50 percent of broccoli is treated and 15 percent of onions (SLUA).“
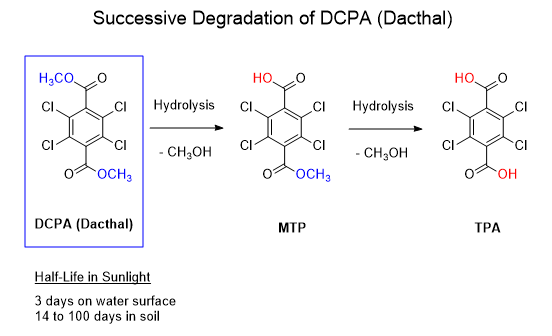
As useful of an herbicide as it may be, it has a dark side that spooked the US EPA into issuing an unusual emergency suspension on August 6, 2024. In particular is the potential toxicity to the unborn and the risk to “post-application workers involved in tasks such as transplanting, weeding and harvesting.” Female farmworkers are at high risk since DCPA has been shown to be toxic to the fetus producing lifelong health problems. The reader is invited to read the link for details in the toxicology. The successive degradation of DCPA is shown below. In addition to hydrolysis, it is also subject to photodegradation in sunlight.
Why wasn’t this discovered earlier? I’m not an EPA pesticide guy, but discovering the specific toxicity of herbicides registered many years previously requires some kind of trigger to get an investigation started. Today, other than an overt incidence of toxic effects making the news, that trigger can be the Registration Review Overview conducted by EPA every 15 years for each registered pesticide.
Having interacted with a certain division of the USEPA for the last 3 years, I can say that there are many intelligent and knowledgeable scientists, engineers and other professionals who try to get things done in a very constricted space bounded by layers upon layers of federal laws converted into regulations. They are about as loved as the Internal Revenue Service and, like IRS, are forced to work wildly understaffed and with an IT system that is decades out of date. A doff of the hat to EPA.