Prologue: I want to give my bona fides on appreciation of the “US space program.” For as long as I can remember I have been a space enthusiast. I followed projects Mercury, Gemini, Apollo, Skylab, X-15, Space Shuttle, ISS, Voyager’s 1 & 2, Cassini and others in real time. Even though space publicists mention scientific research, they never go into more than the very least they can get away with for fear of MEGO- My Eyes Glaze Over. To its credit NASA posts annual lists of research papers with links disclosing research results from R&D conducted in the orbital environment. Here is such a list. Much of the research might seem arcane but it is important to realize that the practical value is likely to come later as others incorporate it into their subsequent research and product development. This is how R&D works.
A few words about Elon Musk’s plans on moving mankind to Mars. As everyone knows, Musk is actively engaged in developing space craft large enough, numerous enough and powerful enough to take a great many people to Mars. His stated dream for humanity is to transport a large number of people to the red planet to establish a permanent settlement- a sort of Earth 2.0 for humans. There is even fanciful talk of terraforming Mars for more convenient and safer occupation. This is a colossal job, even for a small world like Mars.
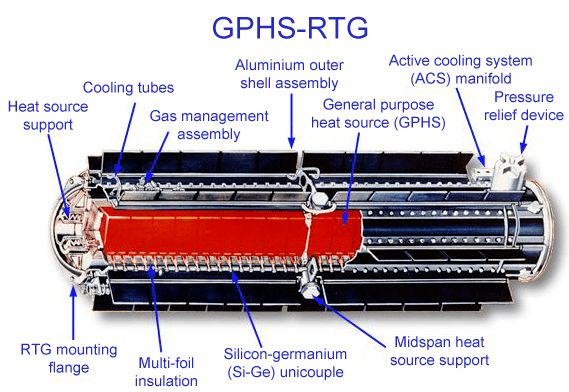
All energy produced and consumed on Mars will be electrical via nuclear energy, solar, or maybe wind (??) generation. Combustion as we know it is out due to the absence of combustible materials and abundant oxygen. Solar power generation will be limited by reduced solar energy shining on Mars and by the practical problem of dust accumulation. Thermoelectric generation from a Radioisotope Thermoelectric Generator (RTG) has been the solution used on many Mars landers and deep space probes.
The best radioisotopes for RTG are alpha emitters. Alpha particles are +2 charged helium nuclei which cause a large amount of ionization over a short distance as it crams its way through matter, stopping in a short distance. Because they lose energy over short distances even in air, alphas require very little shielding, unlike beta and especially gamma radiation.
Betas themselves are easily shielded, but as they decelerate in matter, they can generate radiation called braking radiation, or bremsstrahlung x-rays, which are more penetrating. This is how x-rays are generated in an x-ray tube. Electrons impacting a target like copper generates x-rays. The effect is more pronounced in higher atomic number (high Z) elements like copper, but in low Z materials like plexiglass x-ray generation is much reduced. Consequently, beta emitters are commonly shielded with plexiglass.
The main downside to RTG is the low efficiency in converting thermal energy to electrical energy via the Seebeck effect– about 3-5 % currently according to most sources. So, for every 100 watts of thermal energy production, only 3-5 watts of electrical energy are available. This puts pressure on the supply of scarce radioisotopes.
On the good side of RTGs, they are stable, reliable and long lasting. Waste heat can be used to provide warmth for proper operating temperature in the craft or facility. The Mars lander Curiosity uses 4.8 kg of 238PuO2 to produce 100 watts of electrical power.
The deal with the devil you have to make with RTG power generation is that the best heat generating isotopes in terms of power density (watts/g) also have the shortest half-lives. For instance, 210-Po has a high power density of 140 watts/g but a half-life of only 0.38 years. It undergoes a 5.6 MeV alpha decay directly to stable 206-Pb, emitting a gamma only once in 100,000 alpha decays. Gamma emission poses shielding weight penalties and radiation hazards both in manufacture and operation in space. Even with no humans around, there is still the matter of electronic components that are sensitive to radiation. The more commonly used alpha emitter 238-Pu has a lower power density of 0.54 watts/g but a reasonably lengthy half-life of 87.7 years and minimal shielding requirements.
The background radiation environment in space by itself demands that shielding and radiation hardened electronics be used. Any added radiation from an on-board RTG only compounds the problem. The amount of shielding any given material provides is measured in half-thickness, not “full thickness” and is dependent on the type and energy of the particle. This value is the thickness of a specific material required to reduce the intensity to half of the incident radiation, not the total radiation emerging from the shielding material. This is because scattering can occur within the shielding material contributing to or minimizing the total flux. The point of this is that shielding only attenuates radiation to acceptable levels and not to zero.
238-Pu is a synthetic isotope that must be isolated from other Pu isotopes as well as a dog’s lunch of other elements in spent nuclear fuel or be selectively synthesized by nuclear chemistry. Isotopic separation of 238-Pu from other plutonium isotopes is difficult, slow and not the preferred method of producing it at scale. Nuclear chemistry that provides exclusively 238-Pu from a single transformation as with like 237-Np, offers a more productive route. This allows good old regular, valence-electron chemistry to effect the separation needed.
238-Pu is produced by neutron irradiation of 237-Np producing transient 238-Np with its 2-day half-life and subsequent beta decay to the 238-Pu. Chemical separation of the plutonium from residual neptunium is straightforward but, like all chemistry with radioisotopes, burdened by the need for radiation shielding for safety.
238-Pu is presently in short supply in the US. The Savannah River Site was producing “bulk” 238-Pu but was shut down in 1988. After closing of Savannah, the US purchased 238-Pu from Russia but the word is that Russia is short on it as well. In recent years other sites have been scaling up production where “scaling up” means producing in the several hundred grams to a few kilograms in a campaign.
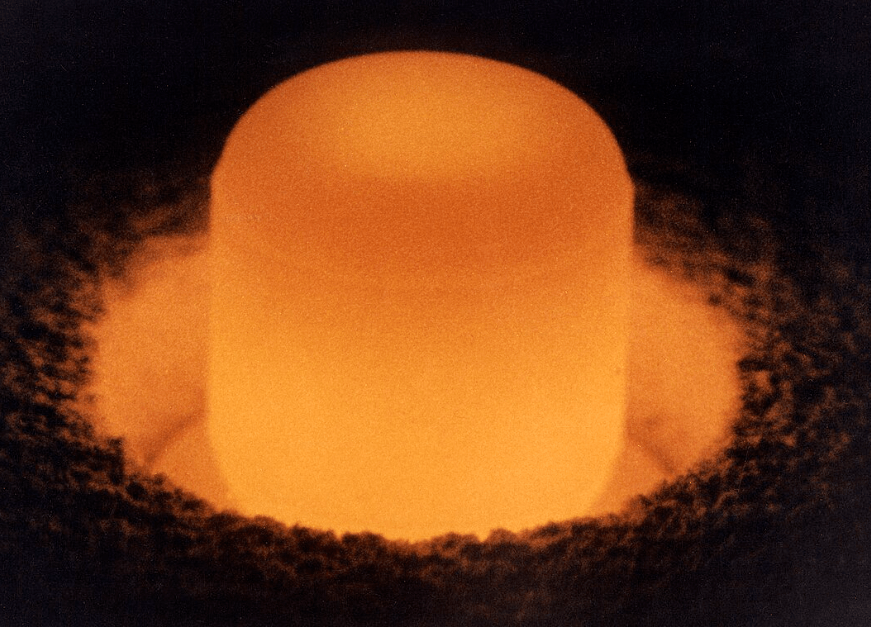
In the RTG, plutonium is not used in the metallic state but as the oxide which is a ceramic or refractory** material like most heavy metal oxides. The plutonium is oxidized to 238PuO2, pelletized and clad in corrosion resistant iridium. According to NASA, this refractory form of plutonium is resistant to an accidental release in a variety of accident scenarios including Earth reentry and rocket propellant fires.

The Seebeck effect is not the only means of producing electrical energy from radioactive decay heat. The free piston Stirling Radioisotope Generator can use decay heat to drive a piston in a Stirling engine using helium gas as the working fluid. Waste heat is dumped at the cooled end of the engine and the linear reciprocating motion of the free piston is used to generate electrical power in the adjacent alternator.
The electric alternator is similar to the electromagnetic flashlight on the market. It works on the ordinary induction principle buy moving a magnet through a coil. You shake the flashlight to recharge it, causing the internal magnet to move back and forth through a coil. Shake it for 1 minute to get 4 minutes of light. The Stirling radioisotope free piston linear alternator operating in this manner can produce 4 times the electrical power of an RGT.
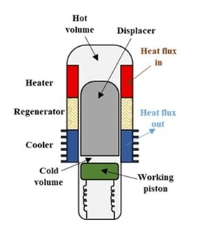
In 2020 workers Wong and Wilson at the NASA Glenn Research Center reported that they were able to operate a Stirling radioisotope power convertor for 14 years maintenance-free.
Off we go!
Some thought will be needed on screening potential migrants to Mars for age, various physical ailments, dental health, genetic predispositions, sociability and underlying psychological issues. A manic crew member could drive fellow crew members to a murderous rage over time. Such screening has been done with astronauts for a long time. I wonder if choosing to migrate to Mars isn’t a sign of some precarious psychological condition in itself, after all the likelihood of a return to Earth may be slim. It would resemble going to jail in some ways.
Over time, the masses of new Martians living in Muskville will have to decide on what to do with themselves beyond exploratory geology, meteorology and engineering studies of Martian accommodations. Mars is a big, arid and frigid desert with no breathable air. But it may offer a few choices for recreation such as spacesuit hiking and shuffleboard. The outdoor choices will be limited by the Muskvillager’s battery, heating and oxygen supplies as well as ability to get around.
Eventually, all manner of psychological, social and physical maladies will manifest in Muskville and will have to be dealt with. People will spontaneously form cliques eventually giving us-vs-them issues requiring mediation. Unless the New Martian settlers are sterilized, pregnancy is a near certainty. An entire book could be written on complications this would bring. The alternative is to limit the inhabitants to a single gender or to gay individuals- most likely a non-starter.
Death on Mars means that your remains will need to rest somewhere outside the facility. A fresh body will freeze stiff in the Martian cold and remain that way indefinitely. Digging a grave will require energy expenditure and digging tools. Cremation will consume considerable power and may be out of reach.
Something like a hospital with medical supplies and trained staff will have to be present. The few physicians who might be present will be required to be generalists with exceptional diagnostic and surgical skills. A full medicine cabinet to cover a range of maladies will be needed to support this.
As Muskvillagers age out, the range of health problems will widen and require care. Diabetes, cancer, dementia etc. will fade in and people will age and die. This will leave job openings and duties behind which will have to be filled.
In general, the conveniences of modern living will be seriously cut short for the New Martians for a long time. A supply line with Earth that can withstand politics, business failure and war must be maintained.
If I were planning a migration to Mars, I’d worry about maintenance and spare parts for everything. Mechanical things will break. Perhaps an orange-colored Home Depot module will hitched to the back of the lander and sent along with a load of duct tape, assorted bolts and screws, sealant, O-rings, hand tools and cleaning supplies. Don’t forget a few bags of peanut M&Ms.
Wherein I jump to conclusions.
The human capacity for folly knows no bound. Woven in with folly are variable education, emotional inputs and diverse belief systems. The migrants will carry religious and political predispositions that they may or may not reveal in screening for candidates. Friends and relatives on Earth will sicken, age and eventually die but access to a return trip to earth may be severely restricted or effectively impossible.
On reflection, establishing even a modest Mars base will involve large energy inputs. Getting to the surface of Mars with enough reserve propellant for the return trip, the establishment of shelter, oxygen and water supplies are the priorities. Beyond just surviving day-to-day, there is interest in the possibility of putting Martian minerals to use as building materials or even water and oxygen production.
There are indications of frozen water on the surface of Mars in certain limited locations. Where there is water there is the possibility of using electric power to produce oxygen. The hydrogen produced may have utility somewhere but its use for combustion seems unlikely due to the corresponding amount of oxygen needed.
Anywhere you have silicates, aluminates and metal oxides, you have oxygen. Silicon and aluminum both have a strong affinity for oxygen and as such represent a thermodynamic well requiring steep energy inputs for oxygen extraction from minerals. Even worse, many silicates and aluminates are oligomers, chain polymers or network polymers that render them insoluble solids with high melting points. Silicates, aluminates and metal oxides are all comprised of a central atom- silicon, aluminum, or a metal -that are electron deficient by virtue of being connected to oxygen anions. In order to liberate oxide from oxidized silicon, aluminum or a metal, something negatively charged needs to come in and displace the oxide species. Metal oxides like the iron oxides are very often refractory requiring high temperatures to react. Then there is a long list of oxyanions like sulfate, phosphate, hydroxide, chromate, ferrates, molybdates, titanates, tungstates, manganates, etc., each with metal cations. After these there are the polyoxyanions …
The point is that there are a wide variety of oxide species to be found in rock and soil with differing properties. In the end, a negatively charged oxide anion must be oxidized to produce molecular oxygen.
A thermodynamic well resides in a substance where atoms come together to form strong chemical bonds and release a great deal of heat into the surroundings. The same amount of energy that was released is minimally what would be needed to drive the reaction in the reverse direction. This bond forming heat energy is dispersed amongst the large number of surrounding molecules. The heat evolved in forming the original bond produces a high temperature locally, but as it spreads out each succeeding layer of neighboring molecules gets a smaller and smaller share of the original energy. As the bond forming energy release is spread over more and more molecules, the resulting temperature rise of the succeeding layers get smaller and smaller.
Newton’s Law of Cooling says that rate of heat flow is directly proportional to the temperature difference between contacting objects. The greater the temperature difference, the more useful work (heat and work are both energy) that can be done. Large temperature differences transfer large quantities of energy. Low temperature differences result in less energy transferred. As the heat spreads to the surroundings it produces decreasing temperatures as the heat conducts away and the ability to do work diminishes. This constrains the recovery of waste heat.
The diffusion of energy in this manner is what entropy is about- the irreversible loss of energy to the surroundings. If you are tempted to talk about entropy, consider that it has to be consistent with its unit of measure: Entropy, S, equals energy per degree Kelvin or Joules/Kelvin (J/K).
In order to get molecular oxygen from minerals it will require a great deal of energy expenditure per kilogram of oxygen. Not only that but specialized equipment and chemicals. Any oxygen produced will have to purified and compressed into cylinders.
MOXIE
The extraction of molecular oxygen from the abundant carbon dioxide atmosphere seems desirable and has actually been put to the test on Mars. A prototype molecular oxygen generator called MOXIE went to Mars on the Perseverance rover and successfully produced oxygen from carbon dioxide beginning in 2021.
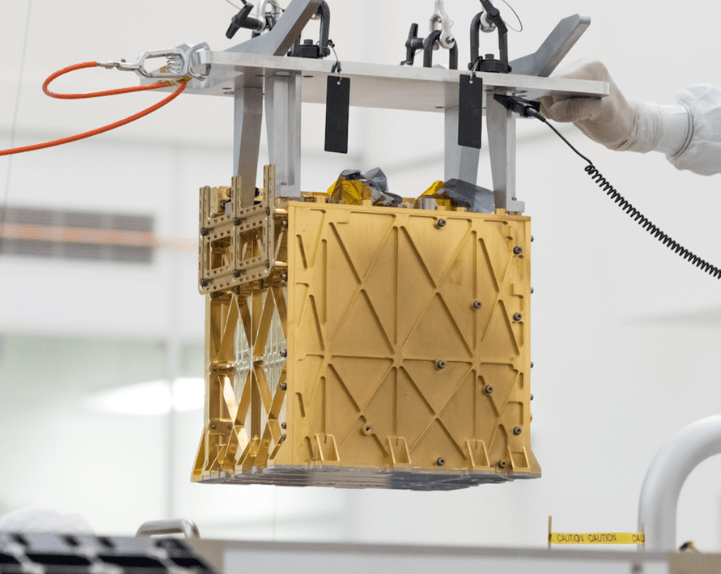
The MOXIE oxygen generator is a solid oxide electrolysis device that operates at 800 oC and uses a stack of scandia stabilized zirconia ceramic electrolyte. An excellent source of information on MOXIE can be found at this Science site.
About 10 % yttria (Y2O3) or scandia (Sc2O3) will prevent the zirconia (Zr2O4) electrolyte from undergoing a phase change that causes the ceramic to fail at high temperature. From personal experience I know that scandia is chosen as a better diluent for zirconia because it allows lower temperature operation than yttria by perhaps 200 oC. The lower operating temperature with scandia allows for better sealing of the cell. High temperature seals are very problematic at these operating temperatures.
The MOXIE electrolysis cell uses a nickel coated cathode for reduction of the CO2, a ceramic zirconia/scandia electrolyte that allows oxygen anions to selectively pass through, and an anode where the anions are oxidized and combine to form O2 where it is captured. MOXIE produced O2 at a rate of 6-8 g/hr while on Mars. The process vents carbon monoxide waste as well as unreacted CO2 at the cathode where it is vented.
A limiting factor in operating MOXIE is the operating voltage across the cathode and anode. Two kinds of chemistry can occur within MOXIE. Carbon dioxide can be reduced to form oxide or carbon, depending on the flow rate of CO2 and the operating voltage. The Nernst voltage, VN, is the minimum voltage necessary to do the chemistry. At about 1.1 volts the cell will reduce CO waste biproduct to carbon on the cathode. This is called “coking”. Carbon formation on the cathode impedes the function of the cathode and reduces the output of the cell. The voltage for coking varies very little with flow rate.
The VN for the desired reduction of CO2 to oxide (O–) and CO at a low flow rate is around 1.0 volts and at high flow rates drops to about 0.95 volts or just a bit lower. So, the “normal” operating voltage range then would be between 1.0 and 1.1 volts to prevent fouling the cathode with coke. The operating voltage window seems a bit narrow. It was found that while a stable operating voltage could be supplied, the resistance of the cell was very sensitive to temperature making stable operation a bit delicate.
Pyrochemistry
Extraction of oxygen from lunar mineral samples has been done previously (below). All of the mineral samples were iron rich and gave yields of 2 to 5 % in the form of water. The samples were from Apollo 17 and consisted of ilmenite (FeTiO3), basalt, soil and volcanic glass. The process uses hydrogen at a reaction temperature of 1050 oC producing H2O. Presumably the water vapor is mixed with hydrogen during and after the reaction. The water can be isolated by simple condensation in the presence of the hydrogen.
Reduction of Ilmenite: FeTiO3 + H2 — > Fe + TiO2 + H2O
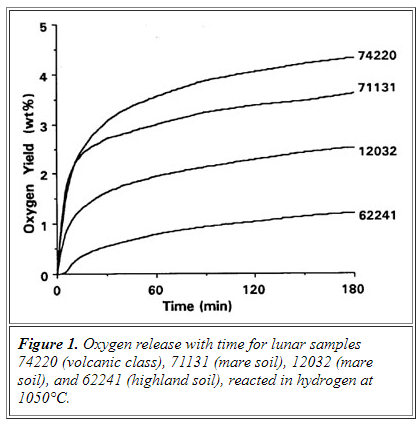
by Carlton C. Allen
Lockheed Martin Engineering and Sciences Co.
To use the process described above, high temperature is required for the hydrogen reduction in a refractory vessel. This requires considerable electrical energy input to heat the thermal mass of the vessel and the regolith. Spent material will have to be removed and discarded. Perhaps the heat can be recovered for general facility heating. Oh yes, the recovered water will need to be electrolyzed to produce molecular oxygen and hydrogen. This process will use plenty of electric power as well as for the compressors to store the O2 in pressure bottles. In principle the hydrogen can be recovered for reuse in the hydrogenation vessel.
The above process applied to ilmenite produces metallic iron and titanium dioxide, a white pigment. FYI, ilmenite is a common raw material for high purity titanium dioxide production. It is high purity because the titanium dioxide is prepared from titanium tetrachloride which is isolated by distillation from the ore matrix after fluidized bed chlorination.
The first Martian settlers will have to bring every single thing necessary to live on the planet. That includes launching it and landing it on the surface intact. Landing on Mars is tricky because the atmosphere is too thin to provide much aerobraking. The Martian surface pressure is the same as the Earth’s at 80,000 ft altitude and the temperatures are frigid.
Let’s say we successfully land a crew and set up housekeeping. What are they going to do with their time? These missions are supposed to last about 2 years including a lengthy transit time. They can collect various kinds of data on Martian geology and weather and send it back to earth. Somebody will get publications out of it. Eventually, somebody will decide that there must be other things to do besides geology and meteorology. Naturally there will be much ongoing R&D on the pragmatics of living on a remote Martian outpost in a crowded pressure can.
Eventually, the question of what non-research living will look like. Shelter will need construction from some kind of materials. Every new section of shelter will need to be airtight and equipped with environmental controls, sanitation and power. Bulkheads between sections will need to be in place to isolate calamities.
Support staff will be needed one day to provide critical services and perform facilities maintenance. This would also include medical staff, emergency care, food & sanitary support, electronics and IT support and administrative staff for the inevitable paperwork. The lander will need rocket engineers for upkeep and repairs to assure launch reliability for the return trip. Do rockets exist that can sit for a year fueled and then reliably launch and insert into a trajectory back to Earth? There are many, many problems to be resolved in many areas.
After some period of time, a crime will happen on Mars. It could be petty theft, assault or even murder. Someone will have to be appointed to look after law and order. An astronaut-sheriff, sergeant at arms or just the po-leese. What kind of due process will be available to a suspect in a Martian colony? Guns will be too risky to have in the settlement given that a bullet could pass right through a bad guy and rip through the structure creating a leak.
On earth, doing independent research requires getting academic credentials, finding a position, grading exams for goddammed freshman chemistry, executing an R&D program, and then going home every day to refresh and have a social life. Imbedded in all of this we have courtships, marriage, mortgages, babies and divorce. We manage the ten thousand details of modern life and interact with our families and social networks. We mourn those we lose and celebrate our achievements. We enjoy good health and suffer injury and sickness and eventual death.
On Mars, the equation will be a bit different. Many of the above life elements will apply, but from a great distance. Instead, we will be confined to a small space with an unchanging group of fellow crew members. The distance to Earth from Mars is constantly changing and there will be a period absent any communication when the earth is behind the sun.
Eventually, research on living in space or on Mars will wind down to minutae if it hasn’t already and people will have to find other things to do. The funding for living off-world will have to switch from R&D to … what, a lifestyle?
I wonder if there will ever be room for commerce and jobs on Mars. I can see running a canteen or restaurant for profit but stocking them with earth supplies will be prohibitively expensive and infrequent.
What joy can there be living in a pressure can on a hostile planet? What few hermit-astronauts there may be might find it acceptable if they never need a dentist. Perhaps dentures or implants should be routinely fitted to all visitors to Mars.
The second stage of Mars exploration will have to ramp up progress on sustainability. Using Martian soil as raw materials for construction and for crops. As the Martian population rises beyond the first few rotating crews, what will the immigrants do with their time in can-living on a hostile world? Would going to Mars to lead an utterly confined life with nothing to do be an attractive draw?
Epilog
I think that settling on Mars is not such a great idea overall and specifically would be wasteful of resources that should be applied to the rehabilitation of the biosphere on our home world. It would somewhat resemble living on the Amundson-Scott Station on the south pole but without the benefits of breathable air or supplies regularly shipped in. Further, the lack of radiation shielding on the surface of Mars will offer 40-50 times the background radiation as on Earth, not counting the occasional storm of angry solar protons the sun flings out now and then.
** NASA does not use the terms “ceramic or refractory” in its description of the 238-Pu heat source. This is my choice of words.

