Attached is an updated reprint of an essay I posted 10/28/07. Since then I have shifted into EPA regulatory compliance within the chemical industry. My views have changed a little, it turns out.
==========
“We live in an age of miracle and wonder” is the refrain from Paul Simon’s album Graceland. All around us and through us are engineered materials devised for their specific physical and chemical properties. Time-released magic bullet drugs that inhibit specific enzymes. Flavors & fragrances, colorants, rheology modifiers, UV absorbers, emollients, preservatives, food irradiation and manufactured food additives are engineered and marketed to satisfy our lizard brain’s willingness to shell out cash-for-fun and stimulate our limbic system’s emotive triggers.
It is hard to avoid contact with manufactured goods that aren’t affected by chemistry. A century and a half of tinkering with substances at the molecular scale has given us the ability to optimize the composition and performance of products that make our lives easier and safer. Microprocessors and Lycra, Hastelloy and Lipitor- the chemical industry has evolved to produce the raw materials and finished goods needed for the performance we have come to expect.
Industry has a Spotty Record of Safety
Along with the considerable list of positive contributions, history provides a detailed record of the problems associated with the exuberant but uncritical acceptance of the flood of manufactured goods. From radium poisoning of watch dial painters to chromium VI to asbestos, there is a long list of accidents, ignorance, negligence and environmental insult. The trail blazing of our chemical industry leaves behind it a chronicle of tragedy as well as benefits.
The result of the checkered past of industry is a growing (some would say “metastasizing”) and intertwined web of state, federal, and international regulatory oversight and requirements. And with it- arguably as a result of it- has come greater institutional risk aversion.
Risk Aversion
In a general way, risk aversion is a type of survival trait and is likely hardwired into our ape brains. It is hard to blame people for being wary or fearful of risks, especially those they do not understand. Over time risk aversion is useful survival trait. But on the other hand, risk aversion is also a type of inertia. Or, it can be a fulcrum from which policy and imaginary justifications are leveraged. The fear of risk may be firmly grounded on experience or it might be imagined or a mixture of the two. The hard part of risk management is identifying real hazards and the probability and magnitude of a bad outcome for managing safety day-to-day. Basically, the hard part is the whole part.
Risk = Probability x Severity of a negative outcome.
Corporate officers have a fiduciary responsibility to the stockholders. They’re purpose is to maximize profits without undue risk to the organization. Most respond to the regulatory environment by perhaps heaving a sigh and relenting to the requirements. Regulatory compliance can have costs associated with it like animal testing of chemical products and intermediates, or engineering upgrades and these costs need to be built into annual budgetary calculations.
How Granular Does Safety Have to Be?
Can safety practices be excessive? I would say that if some specific activity is based on imaginary risks, risks identified by the untrained or massively overestimated risks, the cold eyes of an industry consultant may be needed. Who knows, you may have actually underestimated a risk.
Safety has a large psychological component to it. How do you compel people to behave consistently in a way that keeps everyone safe? Not just immediately, but in the twentieth or five hundredth time they perform a task with associated hazards? Complacency is a normal human weakness where a misstep can lead to casualties.
The amount and type of safety measures in chemical processing required greatly depends on the chemical substance. Some company’s batch records give very detailed instructions to maintain constant safety. Others are less so on the assumption that the operations staff know what they are doing. Too much detail can lead to operator impatience and freelancing.
It is possible for organizations to be dominated by confident voices that are quite risk averse but not very knowledgeable about the technology. Leaders will state that “safety-first is our policy”. A paper storm of SOPs will issue, dragging out the most elementary actions into numerous steps and check boxes. There is great merit to SOPs, but enlightened and proactive interpersonal management of hazardous operations is just as important. Management by walking around works.
Organizations can find themselves spiraling into micromanagement of even the smallest details for fear that the regulatory and liability hammer could fall at any moment. Indeed, if one studies many regulations in detail, it is easy to fall into habit of overreacting. Risk aversion isn’t just a personality issue, it is statutory under numerous regulatory umbrellas.
Being a baby boomer, the chemical safety practices I have been exposed to and have practiced is rather out of date. My education occurred during a time when running chemical reactions on an unventilated bench top was normal. We used Tirrill burners to flame dry our glassware on the sophomore organic lab benchtop and set the hot glass on a Transite square, an asbestos product from Johns Manville. I still would have no problem using Transite. In fact, I have done many things since summer of 1980 that would be frowned upon today. My grad school and post doc time went way into the weeds on using hazardous materials with minimal oversight.
You learn how to safely handle chemicals by safely handling chemicals. In the course of using a chemical, you might conclude that the known hazard of the substance appears to be exaggerated, so over time and repetition you let your safety practices loosen up a bit. Then one day, you cross some unseen line and an energetic event happens. While what you did leading to this outcome might be categorized as carelessness, as they drag you out of the lab by your one remaining arm, your ears ringing, a metallic taste in your mouth, and partially covered by your torn and smoldering lab coat, you recognize that you actually learned something from that experience. You now have a better appreciation for the safe operating range for that chemical. You’ve gotten one quantum unit of expertise. This is one way to gain expertise, albeit a painful one. Perhaps it will be useful once you start your next career. I have had many close calls and each one taught me something. Perversely, not having close calls with chemicals- some would call it luck -actually deprives one of practical and useful experience.
Today I am a senior chemist involved in chemical safety in industry. Until recently, I was involved in finding the thermal safety boundaries of chemical reactions through calorimetry. But with the past experience that I have, I know a bit more about the boundary conditions of handling chemicals than the younger chemists may get to acquire. In order to know how to work with hazardous chemicals you must have worked previously with hazardous chemicals and perhaps seen for yourself what can happen with sloppy technique.
This is nothing reckless like poking alligators with a stick in Florida or free climbing El Capitan. I mean things like seeing what actually happens when you pour concentrated H2SO4 into water fast enough right up to the boiling point taking care not to have a splash. Maybe you can see the heat of dilution boiling the water at the H2SO4/water interface.
The Regulatory Environment
Statutory risk aversion is the domain of the state. The name “Nanny State” is a sarcastic descriptor referring to a perceived excess of regulated requirements and conditions in our lives as well as the set of penalties. Though perhaps well intended, the Nanny State seeks to zero out risk. Even if a situation arises for which there is no explicit regulation, OSHA has the General Duty Clause where employers are required to provide:
“employment and a place of employment which are free from recognized hazards that are causing or are likely to cause death or serious physical harm to his employees…”
This provision exists to address any gaps in OSHA regulations that may not account for unforeseen circumstances. The plethora of regulations is partly due to the vast array of situations in which industrial employees might be injured or killed. Additionally, lawyers have identified and exploited loopholes in the regulations, which are subsequently closed by regulatory agencies. Ambiguities are often resolved through statutory amendments or the application of established case law.
EPA TSCA has the job of generating and enforcing regulations regarding the manufacture and use of a range of industrial chemicals in a limited sector of manufacturing. The central doctrine is from:
As required under section 6(b)(4) of the Toxic Substances Control Act (TSCA), EPA is issuing a rule that establishes a process for conducting risk evaluations to determine whether a chemical substance presents an unreasonable risk of injury to health or the environment, without consideration of costs or other non-risk factors, including an unreasonable risk to a potentially exposed or susceptible subpopulation, under the conditions of use. [Emphasis mine]
TSCA does not include Food, Drugs, Petroleum, Pesticides and a few other areas.
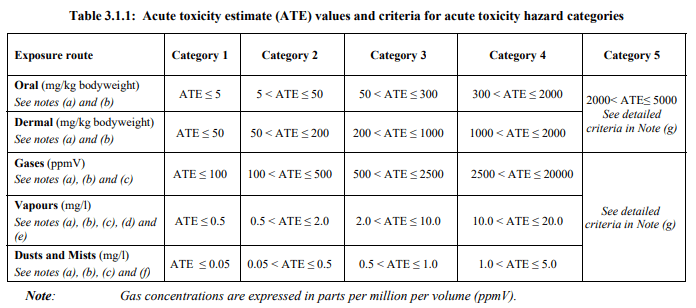
The key words above are unreasonable risk. With every New Chemical Substance filing sent to the TSCA folks at EPA, an assessment must be made by various subgroups for unreasonable risk by the human health group, the engineering group and the environmental group. Thresholds for “unreasonable” have been quantified in order to exclude subjectivity. EPA has many computer models of exposure thresholds, migration in the soil and toxicity to many creatures including humans.
The regulatory environment can make the production of a new chemical substance more expensive or even unfeasible. Nobody advocates the idea that we should be free to pollute and risk the lives of workers and communities. But even for the most skillful and well-intended, there are many regulatory landmines to dodge: air, water, and waste permits; local zoning; OSHA; EPA (TSCA); fire codes; insurance inspections; MSDS’s in multiple languages; ITAR; and DEA. All have reporting requirements, statutes, and paper trails to maintain.
Pragmatics
There are two kinds of disaster that can bring down a chemical plant. One is obviously a fire or explosion in the plant made even worse by casualties. The other is an administrative or legal disaster. This could be a tax problem or worse like having been determined to be out of EPA regulatory compliance for a chemical release into the environment or worker exposure over time. EPA fines are levied per day per violation.
In my view, the USA began ossifying many years ago in regulatory paralysis in much the same way the EU or Japan has. The combination of business risk aversion along with the popular sport of outsourcing our means of production only serves to accelerate the de-industrialization of the USA and the EU. At present there is some effort by the semiconductor manufacturers and others to repatriate manufacturing back to the US out of fear of foreign governments using strategic trade regulation as a competitive cudgel.
What can one reasonably do? Consider even if regulations could be softened, this could take a long time. Until such time as there is a change in regulation, it is best to knuckle under willingly. First on the list is to just be compliant with regulations. Even an excellent argument against an “unjust” regulation enforced by an agency will get you nowhere because regulators are legally required to enforce the regulations and fine violators. If you are facing a regulatory judgement, it is well worth having a lawyer who specializes in that area of the law.
Accepting a harsh judgement on your record can possibly hurt you in the future by having a history of serious earlier infractions. A lawyer can search the case law and possibly find a lesser judgement or better interpretation of the regulations. Avoid at all costs the possibility of being found a repeat violator in some future court action. There could be extenuating circumstances that should be taken into account, but this is the lawyer’s domain and is no place for amateurs.
Fiat Lux
In the chemical industry we have regulatory specialists and EH&S departments who keep on top of the regulations and are responsible for maintaining timely compliance. They help keep the doors open and should be appreciated. That said, executives lurking in the C-Suite should be at least conversant in labor and environmental regulation to the point where they know to get advice before issuing directions relating to this.
Note: Obviously I’m not a damned lawyer and my words should not be construed as actual legal advice. I am simply recollecting learnings from my experiences.