An anti-cancer prodrug called Sirpiglenastat (DRP-104) is in the news. The mode of action is in part the antagonism of glutamine metabolism. In a paper published in Molecular Cancer Therapeutics (DOI:10.1158/1535-7163.mct-22-0282), the authors describe the broader actions of the prodrug-
“DRP-104 demonstrated significant antitumor activity as a monotherapy, which was further enhanced in combination with checkpoint blockade therapies, leading to improved survival and long-term durable cures. In summary, DRP-104 broadly remodels the tumor microenvironment by inducing extensive tumor metabolism effects and enhancing the infiltration and function of multiple immune cells distinct from those obtained by checkpoint inhibitor therapy.”
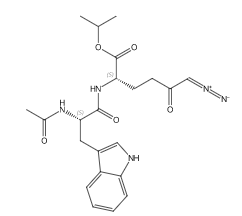
I’m an organikker but not a medicinal chemist. What struck me as interesting is the presence of a diazocarbonyl group on the molecule. This functional group isn’t often found on drug molecules. In the larger scheme of things, there is nothing extraordinary about synthetic chemistry with diazocarbonyl compounds as intermediates for drug molecules, but diazo groups on drugs or natural products is quite a bit less common.
Another paper in the Journal of Clinical Investigation (open access, 10.1172/JCI148550) wrote-
“Broad glutamine antagonism with sirpiglenastat (DRP-104). Sirpiglenastat (DRP-104) is a tumor-targeted prodrug of the glutamine antagonist 6-diazo-5-oxo-l-norleucine (DON), which was identified in the 1950s as a potent anticancer agent (79). DON is a mechanism-based, irreversible inhibitor of all glutamine-utilizing enzymes (80), and thus broadly inhibits metabolic pathways that require glutamine as a nutrient source. The main impediment in the clinical development of DON has been its dose-limiting toxicities to normal tissues (81), especially the gastrointestinal tract, which is highly glutamine dependent (82). By utilizing promoieties that are preferentially cleaved by tumor-enriched enzymes (83), sirpiglenastat is able to deliver DON preferentially to the tumor, increasing its therapeutic index.”

The glutamine antagonist 6-diazo-5-oxo-l-norleucine (DON) mentioned above was isolated from a streptomyces culture found in Peruvian soil. The Wikipedia article mentioned that DON works by alkylation at the active site of the glutamine utilizing enzyme (I’m not happy with this source, but a Google search for a primary reference was not successful). Due to systemic toxicity, DON is not currently in development.
