Jupiter is quite old like the rest of the solar system. But even this far down the timeline, it is still a banded, multicolored gas giant. The same goes for Saturn. How is it that these planets are not some shade of brown or grey? The planet has an active atmosphere with complex circulation patterns. After a few billion years of atmospheric mixing, how is it that Jupiter still has a banded and bespotted atmosphere?
Ever wonder what substances are responsible for the colored features on Jupiter? Molecular hydrogen and helium make up the vast majority of atmospheric components but these gases are not colored in the visible spectrum. Other gases found in the atmosphere include the noble gases argon, krypton, and xenon; ammonia (NH3); methane (CH4); hydrogen sulfide (H2S); water (H2O); phosphine (PH3) are all colorless as well. Ammonium sulfide ((NH4)2S, CAS# 12135-76-1) and ammonium hydrosulfide (NH4SH, CAS# 12124-99-1) are thought to exist there. These last two could arise from a simple acid/base reaction between hydrogen sulfide and ammonia. A more comprehensive view can be had here. From the looks of it, Jupiter is a very stinky place.
The gaseous substances above are certainly colorless when free of suspended particles. Their respective pure condensates while colorless would be expected to produce whitish vapors or liquid/solid condensates. According to one source, ammonium hydrosulfide is a yellow fuming liquid with a boiling point of 51.6 oC at one atmosphere and forms white rhombic crystals under anhydrous conditions. Ammonium hydrosulfide is at equilibrium with its components ammonia and hydrogen sulfide.
Ammonium sulfide is a yellow crystalline solid that decomposes at ambient temperature (and presumably at 1 atmosphere on earth).
Organic compounds like methane, ethane, acetylene, and diacetylene found in trace amounts in the Jovian atmosphere could be activated by UV sunlight in the upper atmosphere into higher molecular weight unsaturated substances that could have visible chromophores present. This would be an ongoing process as circulation moves the substances around so there should be accumulation.
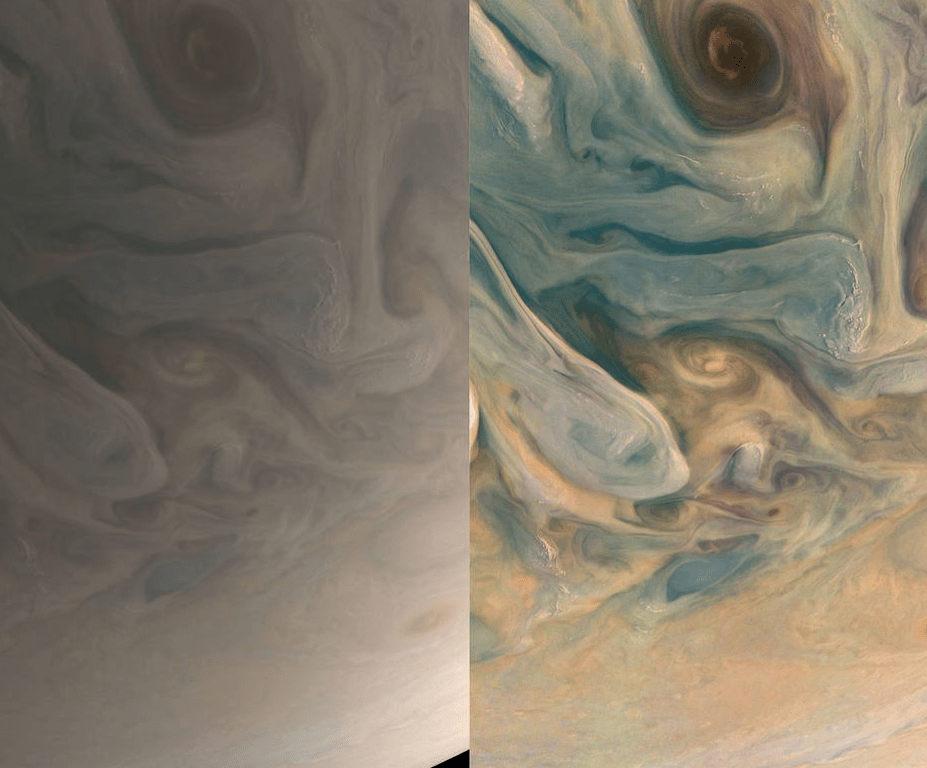
Given the optical opacity of the visible clouds on Jupiter, whatever colors are there must be due to suspended liquid aerosols and solid particulates. The colorful photo below, glorious though it may be, is an enhanced image in the optical wavelengths and possibly suggests there may be a higher concentration of colored substances than really exist.
In fairness, with all imagery, be it chemical photography or digital photography, decisions have to be made about color balance, saturation and contrast. In both cases, be it dyes or silver halide or semiconductor chips, these photosensitive materials won’t be sensitive across the color spectrum in the same way that our eyes are. It is hard to say by just looking at the photos how much image enhancement has been done to them. In particular, how is the color balance established? Well, NASA has made the Juno raw images available to the public so a lot of image enhancement by various people has been done based on aesthetics without regard to visual accuracy.
NASA has a piece of software used for color correction at the link here.
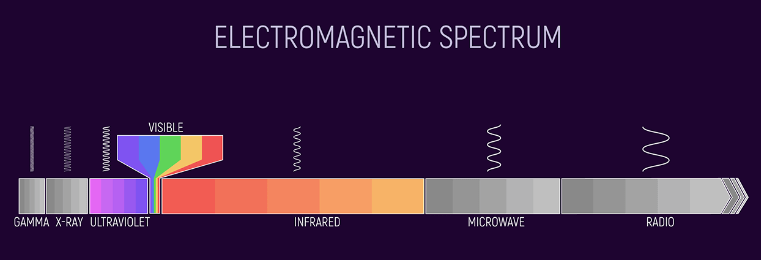
Even more fundamental than the limitations of the sensor chip on board Juno is the matter of “what is color anyway?” In this universe, the color of the spectrum as humans perceive it exists only in the convoluted neural pathways of our brains. In reality, the visible color spectrum is comprised of a band of wavelengths of electromagnetic radiation (EMR) ranging from 380 to 700 nanometers. Every other range of EMR like gamma rays, x-rays, ultraviolet, infrared, microwave and longwave “radio” light could be thought of as having their own “color” spectrum, albeit invisible to our eyes.
A Bit O’Chemistry
Color is a sensation that comes to our consciousness as a result of (bio)chemical mechanisms. Chemistry is generally about what can happen with the outer valence level electrons that buzz around atoms and molecules. We Earthlings are composed of chemicals and because EMR (photons) can interact with substances in ways that depend on the wavelength of the EMR. Our light perception begins with the ability of our chemical building blocks to absorb a certain band of wavelengths. Light can do two things in an encounter with matter- it can undergo absorption/emission or scattering with matter.
Absorption of a photon of visible or ultraviolet light by an organic molecule happens because there is something that can be acted upon to absorb the energy. Absorption of a photon of visible light by a molecule is limited to its valence electrons. In particular, a valence electron can be stimulated to jump to a higher energy level orbital around the organic molecule. This can result in a chemical change in the receiving molecule.
Absorption of infrared light causes vibration in the structure of the molecule. X-rays can cause ejection of inner electrons. Gamma rays can be absorbed or scatter off the nucleus. Microwave photons induce rotational motion or torsion in a polar molecule. Cosmic radiation is often so energetic that molecules are indiscriminately broken at the chemical bond level into neutral or charged pieces, leaving an ion channel along the path of the particle. However, new molecules may form when the reactive fragments recombine. Cosmic ray collisions with atomic nuclei form narrow sprays or showers of nuclear particles as is what happens in earth’s atmosphere. This is called secondary cosmic radiation and is comprised of x-rays, protons, alpha particles, pions, muons, neutrons, neutrinos and electrons.
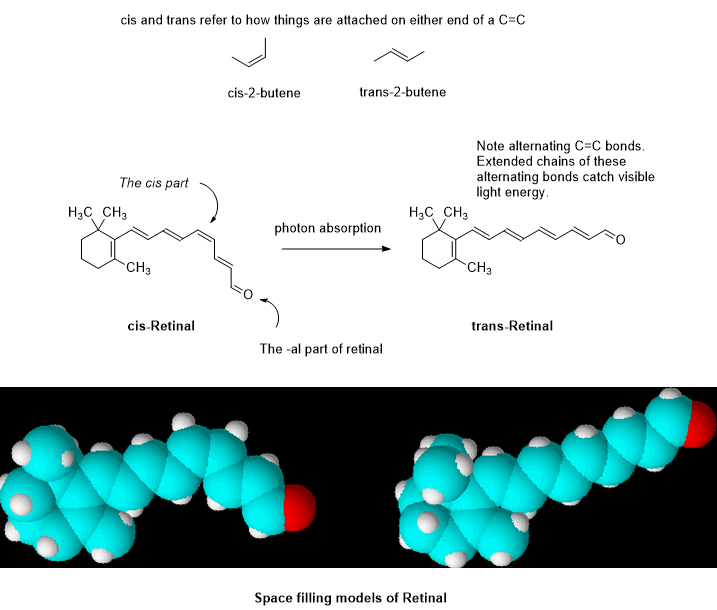
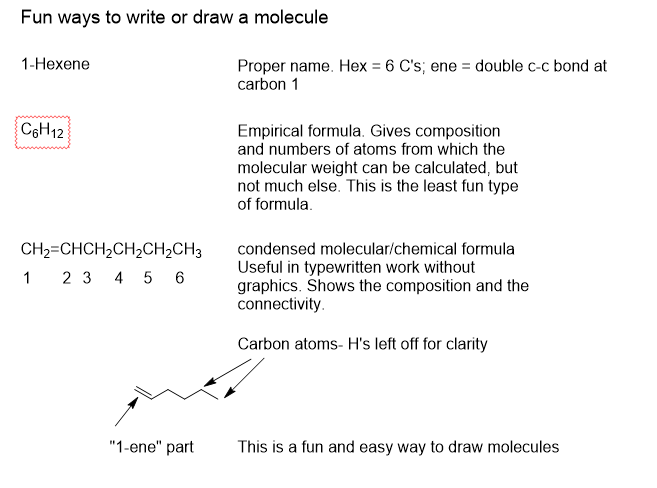
Note the carbon bonds above with two lines between carbon atoms. They are called “double bonds” and they can absorb visible and ultraviolet EMR. When several of them are alternating as in Retinal, they are capable of visible light absorption. Roughly speaking, the longer the chain the longer the wavelength that can be absorbed, not unlike an antenna. Absorption of a photon can cause one of the two bonds to break and allow the remaining carbon chain to rotate about the remaining single bond. In this case the cis form rotates into the trans form which is a bit more stable due to reduced strain energy. The double bond can reestablish in the trans form and lock into place.
In changing from cis to trans, the elemental composition has not changed but the shape and certain chemical and physical properties have. When the shape of a molecule is changed, the manner in which the molecule interacts by contact with other molecules changes, particularly with proteins. This triggers the chain of biochemical events that follow, leading to light perception in our consciousness.
In living systems, some biomolecules have features that lend them the ability to absorb photons, sometimes to a useful end and sometimes to a destructive end (i.e., as with UV light and x-rays). Here, a chemical change would be the rearrangement of an electron around the molecule or a change in molecular shape or both. Receptor molecules in the retina are a particularly good example of a useful result of light absorption.
The result of this change from cis to trans is ultimately communicated from the retina to the brain via depolarization waves moving along nerve fibers and releasing neurotransmitters across synaptic gaps. Importantly, the change that caused the polarization wave is not permanent.
The visible spectrum of light waves, a bit under 1 octave wide, just so happens to be the band of light that can interact with valence electrons absent the destructive excitation that UV and x-rays cause. Infrared light causes vibration of chemical bonds and microwaves cause rotation of polar molecules. Longer radio waves pass right through us.
Rather than go into the biochemistry of this I will invite the reader to surf the interwebs for more. When you examine the chemical mechanism of light perception, think about what it took to figure this out.
Back to Jupiter.
Well, something opaque and colored is swirling around Jupiter persistently- just what the heck is it? The above example of Retinal was of a carbon-based, organic substance. The way carbon-based molecules interact with light is somewhat different than inorganic complexes. Whereas organic molecules can have double bonds and lone electron pairs that can interact with EMR, inorganic substances are largely absent this bonding feature. Instead, absorption and excitation of valence electrons and the net charge of a metal ion are involved. Inorganic substances as a group have a very broad range of colors.
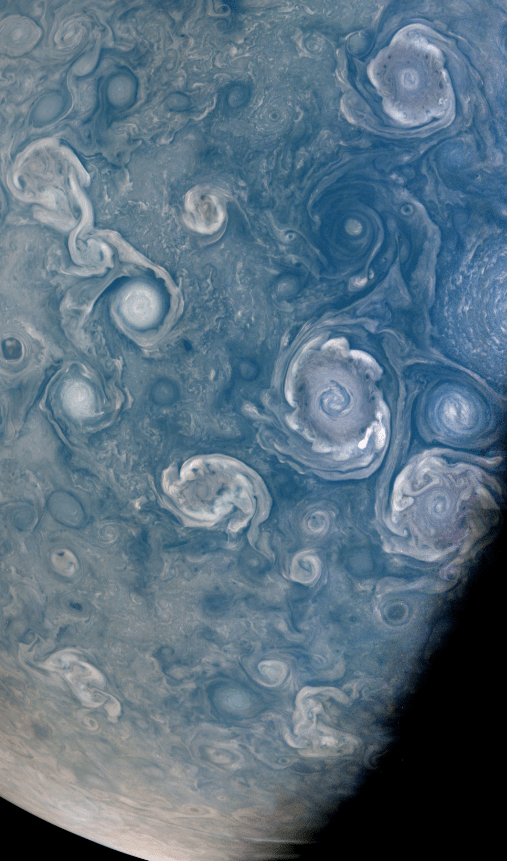
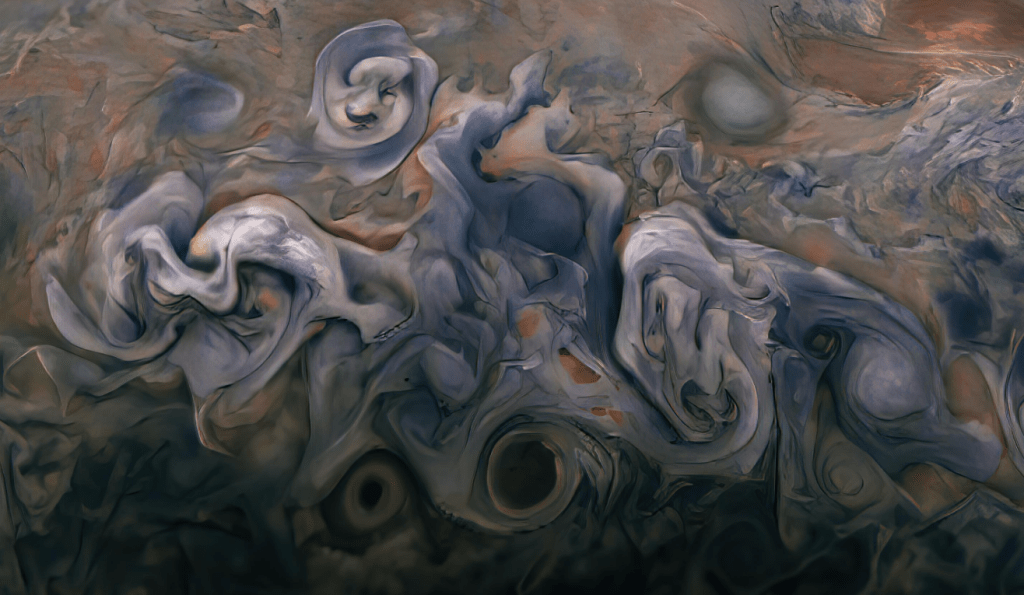
What is of interest here is why the atmosphere hasn’t mixed into a single color over cosmic time. By visual inspection of the Juno images, Jupiter’s atmosphere is covered with abundant turbulent flows in the atmosphere.
The answer must relate to the unseen vertical flows. A colorless gas that condenses into clouds transitions from colorless to opaque as it rises, cools and condenses just like on Earth. Jupiter is famous for its colored stripes and the persistent Great Red Spot. These stripes render visual certain flows around the planetary axis. Due to the spherical shape of the rotating planet and heating from the sun, there will be a temperature gradient with altitude, a gradient pole to equator and Coriolis effect. All of this with varying amounts of vertical mixing as well.
There must be the possibility of non-gaseous material being lofted into the atmosphere from some liquid or solid surface below into a stable but complex system of circulation patterns. The process would self-select the finer particulates that are small enough to remain suspended in the atmosphere. But this in itself does not explain the presence of the colored bands or swirls.
Perhaps the colored bands and swirls infer a solid or liquid surface below that is inhomogeneous, that is, there are localized enriched “deposits” of particular substances. These surface deposits may or may not be “locked” into the latitude by the prevailing winds according to the physical properties of the material.
The apparent longevity of the multicolored atmosphere could be because the striped, large-scale circulation features are of sufficient strength that their inertia carries them around the planetary axis and directs them away from latitudinal flow. This would not prevent vortex formation at the interface or even within the band.
Enough. This is where I get off the hamster wheel of wild scientific speculation.
A few details on the JunoCam can be found here.
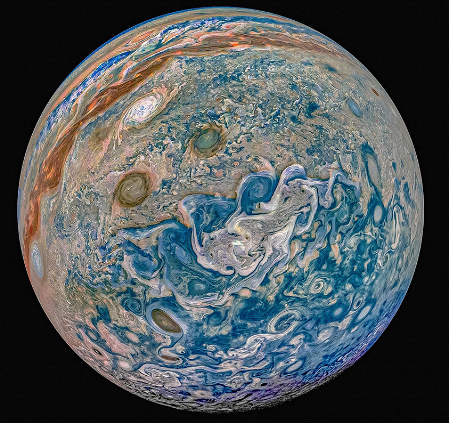
The above image is spectacular but is not what the human eye would perceive. Below is a comparison of a simulated human eye view vs a processed image with increased color saturation and contrast.