[Note: This post is about replacing the hydrogen atoms along the carbon backbone of a polyolefin polymer with fluorine atoms to produce a fluorocarbon surface on a finished good. Here “finished good” refers to anything from polyolefin pellets, powders, components or blow molded articles such as HDPE bottles.]
Recent news has highlighted the use of fluorinated High-Density Polyethylene (HDPE) packaging for pesticides and other products, bringing more attention to the issue of PFAS/PFOS contamination.
What we’re not talking about is a polymer made from fluorinated monomers or comonomers. This refers to a hydrocarbon HDPE bottle made from ethylene (H2C=CH2) monomer that is fluorinated after the bottle is manufactured.
What’s more, the HDPE fluorination process is said to produce PFAS/PFOS (how?) substances that can migrate. Although this technology is not new, and fluorinated hydrocarbon bottles have been around well before the widespread concern over PFAS/PFOS residues, the significance of such contamination was not fully anticipated. As a chemist, the extensive release of fluorinated low molecular weight alkyl derivatives like PFAS/PFOS came as a surprise to me despite knowing that an analogous situation with fluorinated pharmaceuticals that are getting through wastewater plants due to their resistance to microbiological decomposition. For myself only, very little concern for PFAS/PFOS pollution has been noted. You might suppose that chemists could have led the way to understanding. But, not to my knowledge.
The perfluorinated alkyl materials in question bear a close resemblance to TeflonTM which is known for its chemical inertness and lubricity. In chemistry, Teflon is usually ignored as unreactive with most chemicals, except perhaps molten alkali metals. Strategically placed fluorinated features on a molecule can lend the property of greater hydrophobicity or lipophobicity with increased electron withdrawing properties. The high electronegativity of fluorine pulls electron density towards the fluorine atoms through the sigma bonds of a molecular skeleton. Fluorinated organic acids very often have dramatically increased acidity like triflic acid, CF3SO3H, or increased alkylating reactivity like magic methyl, F-SO2(OCH3). By contrast, fluorinated carbon chains themselves are fairly unreactive and quite hydrophobic, as in water repellant. The water repellency of fluorinated hydrocarbons is a very attractive property commercially.
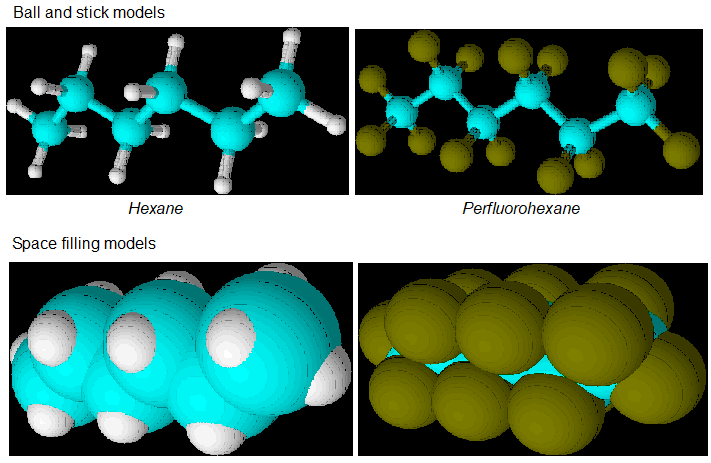
Below are images of the hydrocarbon hexane in ball and stick form and below in a space filling rendering. To the right is perfluorohexane and below that is its space filling rendering. Hexane is just an example of an “ordinary” hydrocarbon that could be perfluorinated.
A brief interlude on molecular polarity
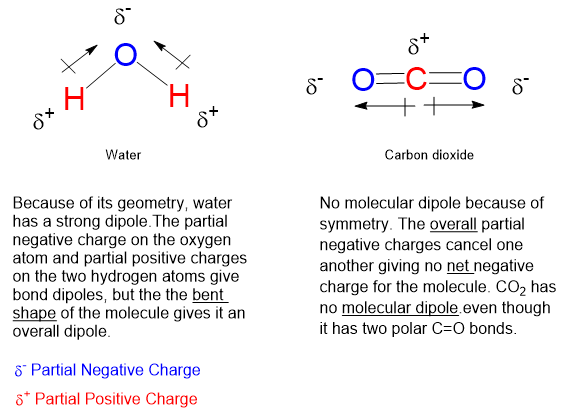
Before we go on, there is the matter of polarity, dipolarity, dipolar chemical bonds and dipolar molecules. A dipolar polar chemical bond is one in which the distribution of electrons is lop-sided. That is, one atom of a chemical bond has a bit more negative charge than the other, which is thereby deficient in negative charge, or by default carrying a partial positive charge. Chemical bonds, functional groups and entire molecules can be dipolar.
But charge comes in whole numbers, so how can we talk about partial charge? A covalent chemical bond consisting of 2 atoms, same or different, will hold together because the two atoms share a pair of outer electrons. If one of the two atoms in the bond has a greater affinity for negative charge, then the cloud of 2 bonding electrons will spend a bit more time near the more electronegative atom. This shift leaves the other nucleus slightly deficient of negative charge averaged over time meaning that the positive charge of the nucleus is slightly more exposed to the world.
In chemistry there is a saying- “likes dissolve likes”. This means that a polar solvent like water can more readily dissolve polar solids and may mix freely with other polar liquids. Nonpolar liquids like hydrocarbons can dissolve nonpolar solids and may mix freely with other nonpolar liquids. Amphiphilic substances have both polar and non-polar features allowing them to compatibilize polar and nonpolar molecules together. Soaps and detergents are in this category.
We should be careful here. The polar-polar and nonpolar-nonpolar solubility generalizations above are really just bookends across a vast open shelf of partial solubilities between them. Nonetheless, it is a useful rule of thumb.
So, if likes dissolve likes, and the fluorine atoms on a molecule accumulate a bit of negative charge, then why doesn’t a fully fluorinated organic molecule freely dissolve in water owing to fluorine’s negative polarity via hydrogen bonding with water’s positively polarized hydrogen atoms?
Carbon atoms can form bonds with itself or other atoms in several ways that give rise to different overall shapes.
Back to our regularly scheduled content
In situ fluorinated packaging, a niche within the packaging industry, was not something I was fully cognizant of until recently. I have come to understand that HDPE, along with numerous other polymers, can undergo treatment with elemental fluorine or fluorinated reagents to alter the hydrocarbon polymer’s C-H groups and convert them into C-F groups. This alteration gives the HDPE surface properties similar to a perfluorocarbon like Teflon™. For HDPE pesticide packaging, this fluorocarbon layer reduces the product’s permeability to the pesticide’s components. Package fluorination is all about reducing permeability of the container.
Definition: Hydrocarbon. A category of substances composed only of hydrogen (H) and carbon (C). There are 4 main sub-categories: Alkanes, alkenes, alkynes, and aromatics. A hydrocarbon can be composed of any combination of the 4. The principal mineral sources of hydrocarbons are coal, petroleum and natural gas. A hydrocarbon may also be called an organic substance, where organic refers to a carbon-based substance.
HDPE, high density polyethylene, is a hydrocarbon polymer of ethylene gas and often with various hydrocarbon comonomers. Hydrocarbon polymers, also called polyolefins, are notable for their considerably inert chemical properties. Inertness is the resistance to chemical change. However, contact with certain fluorinating agents like F2, ClF3, NF3, etc., diluted in an inert gas can, at relatively low temperatures, exchange the H atoms of HDPE with F atoms. Eventually, all or most of the H atoms on the polymer surface will be exchanged. A carbon molecule that has F atoms replacing all H atoms is said to be perfluorinated.
Pesticides are meant to be spread over selected parts of the environment to do their trick. A great many pesticides are synthetic organic chemicals so naturally there is the possibility of any given pesticide or solvent to diffuse through a hydrocarbon-based container. Migration of product molecules into the polyolefin packaging, in this case (HDPE), can result in the release of the hazardous contents and compromise the overall containment, possibly resulting in exposure to the public and the environment.
It should be possible to slow the rate of diffusion of any given hazardous material through a non-fluorinated container by simply making the container walls thicker. The polyolefin manufacturers would be in favor of this, but the converters who buy the plastic pellets to blow mold the containers may balk. Their raw material costs would rise and they would have to pass the costs to customers, who will resist the cost increase. Then with the increase in mass flow of polymer melt necessary, perhaps the throughput or required extruder torque might change unfavorably. Hard to say.
Some of the small-molecule bad actors
On March 5, 2021, EPA published the list below of PFAS/PFOS compounds found in the 20-50 ppb level in fluorinated HDPE containers used to store and transport a mosquito control pesticide product.
| Abbreviated | Full Name |
|---|---|
| PFBA | Perfluoro-butanoic acid |
| PFPeA | Perfluoro-pentanoic acid |
| PFHxA | Perfluoro-hexanoic acid |
| PFHpA | Perfluoro-heptanoic acid |
| PFOA | Perfluoro-octanoic acid |
| PFNA | Perfluoro-nananoic acid |
| PFDA | Perfluoro-decanoic acid |
| PFUdA | Perfluoro-undecanoic acid |
These are all perfluoroalkyl carboxylic acids listed by increasing chain length. Notably the terminal carbon is fully oxidized to the carboxylic acid and is not fluorinated. This acidic end gives a chemically reactive handle for further manipulation of the PFAS/PFOS if desired.
PFOA, perfluorooctanoic acid, has been industrially produced by what is now 3M since the mid-1940s. It has been used to place TeflonTM coatings on frying pans. It was originally prepared by the electrochemical fluorination (ECF) of octanoyl (ock TAN oh ill) chloride, the hydrogen saturated 8-carbon acid chloride. ECF produces the perfluorooctanoyl fluoride which is then hydrolyzed to the acid chloride liberating HF.
Perfluorination of HDPE bottles relies on the most electronegative element, diatomic fluorine gas, F2, or other similarly reactive fluorinating reagents, and does chemistry on a solid polyolefin surface. Fluorine gas is diluted in a suitably noninterfering gas like nitrogen, argon or CO2 and then exposed to the polymer of interest at a prescribed pressure, temperature and exposure time. Fluorine atoms replace hydrogen atoms on the polymer chain. According to one source, the rate of fluorination is diffusion limited. This means that the fluorination reaction is very fast. The presence of molecular oxygen with molecular fluorine had a retarding effect on fluorination proportional to the concentration of oxygen gas. The presence of oxygen led to it being incorporated onto the polymer.

Given the advantage of impermeability provided by fluorinated polyolefin articles, it is clear that there are many excellent applications of in situ fluorinated polyolefins. The replacement of glass and metal with lighter fluorinated HDPE containers may save on transportation costs on a weight basis. Whether or not the economics favor fluorinated polyolefins over glass or metal manufacturing costs kg for kg is unclear.
The range of application categories listed above is quite large. Each entry in the list has many individual components that may be subject to fluorination as well. It is no wonder that PFAS contaminants are spread widely around the world. The US EPA has issued a letter (below) to companies fluorinating HDPE to beware of accidentally producing PFAS/PFOS in their operations. Specifically warning about the connection of PFAS formation caused by the inclusion of oxygen in the fluorination process. The letter specifically cites “EPA’s 2020 long-chain perfluoroalkyl carboxylate (LCPFAC) Significant New Use Rule (SNUR) (40CFR § 721.10536), that are found to be present in or on fluorinated polyolefins may be subject to TSCA regulations and enforcement.”
“It is during certain types of fluorination (e.g., the presence of oxygen) that the manufacture of PFAS has occurred. Manufacturers (including importers), processors, distributors, users, and those that dispose of fluorinated HDPE containers should be reminded of this potential for manufacturing PFAS and comply with any applicable regulations under TSCA, as described in the next section.“
“EPA is aware of alternative fluorination processes that use fluorine gas in the presence of gaseous inerting (e.g., nitrogen) without the presence of oxygen that could reduce the potential for unintentional manufacture of PFAS. These alternative processes for fluorination of polyethylene are highlighted in the U.S. Food and Drug Administration’s (FDA) August 2021 letter on this issue as it relates to food contact articles.”
“Requirements under TSCA PFAS Significant New Use Rules. Certain PFAS, including long-chain PFAS as
defined in EPA’s 2020 long-chain perfluoroalkyl carboxylate (LCPFAC) Significant New Use Rule (SNUR) (40
CFR § 721.10536), that are found to be present in or on fluorinated polyolefins may be subject to TSCA regulations and enforcement. EPA considers the manufacturing of certain PFAS from the fluorination of polyolefins to be a significant new use under TSCA. LCPFAC chemical substances present in polyolefins due to the fluorination process would be considered byproducts of the manufacturing process because they are produced during the manufacture of the fluorinated polyolefins and do not have a separate commercial intent (40 CFR § 720.3(d)). LCPFAC chemical substances that are byproducts of the manufacturing process for fluorinated polyolefins do not meet the requirements of the byproducts exemption at 40 CFR § 721.45(e)5 and are subject to significant new use notice requirements. Significant new use rules require industry to notify EPA at least 90 days before commencing the manufacturer including import or processing of subject chemical substances for a significant new use. The required significant new use notification (SNUN) initiates EPA’s evaluation of the conditions of use associated with the significant new use. Entities may not commence manufacturing (including import) or processing for the significant new use until EPA has conducted a review of the notice, made an appropriate determination on the notice, and taken such actions as are required in association with that determination. Tala R. Henry, Ph.D., Deputy Director Office of Pollution Prevention & Toxics 2022/03/24.”
Fluorination and fluoridation. What’s the difference?
So we do not make people worried about their fluoride toothpaste or their fluoridated drinking water, let’s sort this out. Toothpaste and drinking water have a soluble ionic fluoride salt like sodium fluoride, NaF, or sodium monofluorophosphate, sodium MFP or chemically Na2PO3F. Sodium MFP is water soluble but not stable in water. It hydrolyzes to release fluoride by displacement by water to form dibasic phosphate. The MFP hydrolysis reaction is: PO3F2− + HO– → HPO42− + F−. The fluoride anion, F–, is not nearly the same as fluorine gas, F2. The F– ion bumps into tooth enamel where it binds tightly with calcium in the tooth: Ca5(PO4)3+(aq) + F−(aq) → Ca5(PO4)3F(s). This is the context in which the word “fluoridation” is used. Fluoride ions bond tightly to calcium++ ions in general. Fluoridation is just a specialized variety of fluorination and is mostly confined to the area of water treatment and toothpaste.
Fluorination is a chemical process wherein fluorine atoms are added to chemical compounds. Contact between organic substances and pure elemental fluorine gas is extremely exothermic and sometimes explosive. The dilution of F2 gas with an inert gas like nitrogen, helium or argon has a thermal safety component as well.
Polymer fluorination out in the world- Patents
One source of manufacturing information about proprietary articles and processes is the US Patent and Trademark Office, USPTO. In order to secure your legal right to a patent, the patent applicant must disclose the exact art that is being claimed. This is because the world must have a fair chance to avoid infringement. Google Patents provides the exact text of individual patents, US and others. It also provides a timeline showing the ownership of the patent and whether or not the patent is active, expired or abandoned. Google patents also provide links to patents cited in the patent and patents that have cited the instant example.
Being a Google product, Google Patents has extensive and flexible search capacity. Rather than attempt to make a list, it is a better use of the reader’s time to go to the site yourselves and explore. Note that a search will find patents from all over the world as well as patent applications. Google patent provides a English translated version of the patent.
In searching for patents claiming compositions and methods around the fluorination of polymers, more than a few patents can be found. One can search for patents using the USPTO website (obviously) or from Google Patents.
Another good place to look for relevant art is from a patent you have already pulled up in Google Patents. Near the bottom of the patent from Google Patents is a section labeled “Patent Citations.” This section list prior art patents disclosed by the assignee and those found by the patent examiner in the course of the examination process. Prior art is disclosed by the assignee in the granted patent as well, but in Google Patents there are hotlinks to patents to aid the convenience factor.
In situ fluorination
There are companies who will fluorinate the surface(s) of High-Density PolyEthylene (HDPE) and PolyPropylene (PP) containers. HDPE and PP are especially of interest owing to their utility in packaging liquids. These two polymer classes have great rigidity and strength and are in wide use. However, they share certain weaknesses such as air permeability and permeability of the contents. Air permeability is highly undesired in food packaging as it allows for reduced shelf life or customer satisfaction with the contents. Food and drugs may be susceptible to air degradation and possible reduction of shelf life.
Note: The expiration date on a product does not necessarily mean that the product will go bad when that date arrives. The day after the expiration date is that date at which the manufacturer/seller will no longer guarantee “freshness” or some other type of quality. For instance, normal pasteurized milk is not sterile. Pasteurized milk should be good up to 1 week past the code date as long as it has not been allowed to warm up or been contaminated. Once the milk has warmed to room temperature, the normal bacteria loading will enter log-phase growth and could spoil within 1 day.
In situ fluorination is process wherein hydrocarbon polymer containers are exposed to diluted fluorine gas at a specified temperature for a specified time. At the surface hydrogen atoms along the length of the polymer are replaced with fluorine atoms. The result is a polymer along the surface which resembles TeflonTM to some extent. Some of the desirable properties of TeflonTM are then taken on by the HDPE or PP surface. This H/F exchange at the surface does not affect the properties of the base polymer.
There is one caveat, however. The fluorination must be performed with the exclusion of oxygen. One source says that the vacuum chamber in which the fluorination will take place must be pumped down to 0.1 Torr of residual air prior to exposure to fluorene gas.
Fluorination patents
Below us from the description in US5274049A Filing date 1991-07-19, Application filed by SHAMBAN WILLIAM S, W S SHAMBAN AND Co.
A method for the direct fluorination of elastomers “in order to reduce the static and dynamic friction characteristics and to increase the wear life and abrasion resistance of the elastomers. The invention also relates to elastomeric articles modified by the fluorination method.”
“What is claimed is:
1. A method of producing fluorinated elastomeric articles, consisting essentially of the following steps:
providing an elastomeric article, said elastomeric article comprising an elastomeric polymer having a backbone chain having a plurality of hydrogen atoms attached thereto; and
exposing said elastomeric article to gaseous fluorine under conditions sufficient to reduce the friction coefficient of said article without promoting degradation of the tensile properties of said article.”
Claim 8 claims a method using a hydrogen fluoride scavenger …
“8. A method for producing a fluorinated elastomeric article having a reduced coefficient of friction, comprising the steps of:
placing a thermoset elastomeric article and a hydrogen fluoride scavenger in a closed reactor vessel, said thermoset elastomeric article comprising an elastomeric base polymer having a backbone chain, said backbone chain including sufficient carbon atoms having replaceable aliphatic carbon-hydrogen bonds so that a fluorinated matrix of said fluorinated elastomeric article reduces said coefficient of friction;”
In the description the patent cites sodium fluoride, NaF, as an HF scavenger wherein NaF + HF => Na[HF2], sodium bifluoride.
Inhance Technologies LLC filed application US20190040219A1, but it was later it was abandoned due to failure to respond to an office action. The application claimed a multistep method for fluorinating elastomeric workpieces with 20 % F2 in nitrogen and “altering certain mechanical properties such as tensile property [and] the elastic modulus, an impact property, a wear property, etc.“
Systems and methods for processing fluoropolymer materials and related workpieces, US11879025, filed 2021-04-23, Current Assignee: Inhance Technologies LLC. Claims method of removing perfluorinated compounds from fluoropolymers. The core of the art involves placing a fluoropolymer work piece in a thoroughly deoxygenated chamber, heated from 25 C to 300 C and exposed to a fluorinating atmosphere such as F2/N2 for specified time period. This treatment is claimed to remove fluorocarbons like PFOA to non-detectable levels. There is no mention of where the PFOA goes afterwards, but it looks promising if accurate. However, the granted patent is off-limits for 20 years unless a license is obtained or some other arrangement is made.
Fluorination is imbedded deeply into the design of a great many articles of commerce. The water repellency of perfluorinated polymers in fabrics is one of the chief applications of fluorinated organic materials. The inherent lubricity of PTFE, its built-in chemical inertness and its hydrophobicity have ingratiated millions of consumers and have met performance expectations world wide.
Perfluorinated foams for fire protection in aircraft hangers and industrial spaces are valuable for their ability to float on the surface of burning liquid fuels, blanketing the surface as a vapor and oxygen barrier. The suppression of flammable volatiles in a fire by a layer of protective foam can inhibit flashover of the fire, reducing the overall damage of a fire. The fire retardancy of perfluorinated substances inhibits their combustion and discourages continued burning when the flame source is removed. Halogens as a group have been used for fire retardancy and with bromine in particular.
The chemical origin of the fire retardancy properties of perfluorinated organic materials lies in the low reactivity of the -CF2– fluorine atoms with oxygen. In the combustion of hydrocarbons, hydrogen atoms are readily removed by oxygen or radical species to form water. The C-F bond is one of the strongest bonds in organic chemistry and is slow to be removed by oxygen.
Drug molecules are frequently fluorinated in particular locations on the drug molecule. A C-F bond resists catabolic degradation and enhances the local hydrophobicity of the drug allowing for greater half-life and enhanced drug potency. The down side is the resistance to catabolic degradation and excretion. Many drug molecules are released intact into sewage treatment facilities where they also resist degradation, possibly due in part to the fluorinated features. The effect is that fish and other organisms are exposed to the drug. As with humans, fish and other creatures of the waterways and soil did not evolve with biochemical mechanisms to deal with fluorinated organics.
In the in situ fluorination process, PFAS/PFOS side products can form, especially when oxygen is present. This can be monitored by quality control but companies will comply with recommended PFAS/PFOS best practices only if there are regulations or the threat of them. Nations regulating PFAS/PFOS contamination will have to compete with nations who do not impose regulations. This is the usual scenario for nations with heavy reliance on imported articles but uneven regulation.