Note: What follows are my observations and information from my oncologists and what is scraped off the interwebs. I try to seek information from either primary research literature, medical textbooks or from credible secondary sources. For treatment, I stick to a university medical institution and medical school faculty managing my treatment. I tend not to believe in dietary or nutraceutical approaches. It has been my observation that the origins of cancer are biochemically different from curative or preventative biochemistry. In other words, preventative measures by diet or supplements are mechanistically distinct from the treatment of cancer cells. Divine intervention is not testable, driven by faithful wishing and is supported only by anecdote. I believe that if something truly happens in the universe, it will have an observable mechanism and therefore be measurable.
Oh yes, if you’re squeamish with talking about your prostate because it’s part of your reproductive apparatus, get over it. Part of successfully living with cancer is being able to talk about and learning from it. I’d rather die at least knowing about it.
Because of modern medicine, my experience with both throat and prostate cancer has not been a rocket sled ride to the hereafter. It’s been said that some cancers can be thought of as a treatable, chronic condition and for me that has been true thus far. As luck would have it, my throat cancer was viral in origin and consequently highly treatable by IMRT irradiation and cisplatin. Since 2013 I have had yearly checkups that have all indicated no visible return of the cancer. Since I go to a university medical center, I have had medical students and various head and neck residents also peering down my throat from a camera threaded through my nose picturing my gullet in all of its pink glistening majesty.
The prostate story is a bit different. Before diagnosis the cells had already left the prostate (stage 4) and were judged to be Gleason 9 by histopathology. This was unfortunate. Outside of the prostate capsule they began to wander around through the lymphatic system, lodging in the lymph nodes. Since there was no unified target for surgery or concentrated radiation, The cutters were not called in. Elvis had left the building. After IMRT radiation of the prostate, seminal vesicles and suspected nearby lymph nodes along with 2 years of hormone ablation, my PSA returned to 0.01 ng/mL. Things had taken a turn for the better.
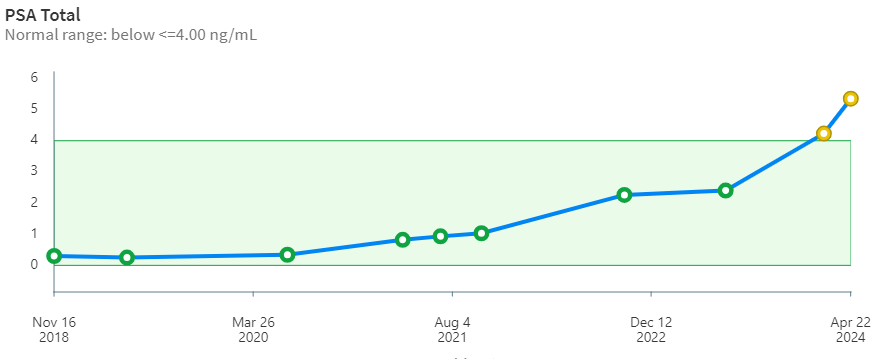
But, the other shoe had to drop eventually. After 9 years, my stage 4 prostate cancer has begun to ramp up steeply. The PSA curve over time (below) is looking more and more like a hockey stick. The borderline PSA value for treatment is 4.00 ng/mL. When it pops up over that value the oncologists begin to take notice. Whether this is based on some statistical mortality data or because of what insurance companies will likely cover is unclear to me. Importantly, PSA may also indicate non-cancerous conditions like prostatitis and benign prostatic hyperplasia. PSA is only an indicator and alone is not definitive. Biopsy is needed to verify and grade the tissue. Of this whole adventure, the biopsy was the worst of it for me. During the procedure, the urologist asked questions about my hobbies -his was carpentry- but I was too distracted to talk about airplanes.
Stage 4 is indicated by histology and backed up by the PET scan revealing radioactive (avid) spots outside of the prostate. Thankfully, this time around nothing was found in the head & neck, chest, prostate or bones. That was good news.
However, the PET/CT scan did show the presence of 5 or so avid lymph glands along the aorta from below the chest to above the prostate.
A proper prostate cancer diagnosis requires more than just a PSA value. An abnormal prostate is detected by digital examination by a urologist and the presence of cancer cells is confirmed by biopsy by a histologist.
But, wait a minute. Exactly what is PSA and what does it do? According to Wikipedia, Prostate Specific Antigen (PSA) is a peptidase enzyme (a protein) secreted by the epithelial cells of the prostate gland. It’s immediate job is to liquify the semen in the seminal coagulum, allowing sperm to swim freely. It is also thought to be involved in dissolving cervical mucus, allowing sperm to enter the uterus. Amounts of PSA above a certain threshold are not normally found in the blood. Elevated PSA is associated with prostate cancer. It’s just a marker.
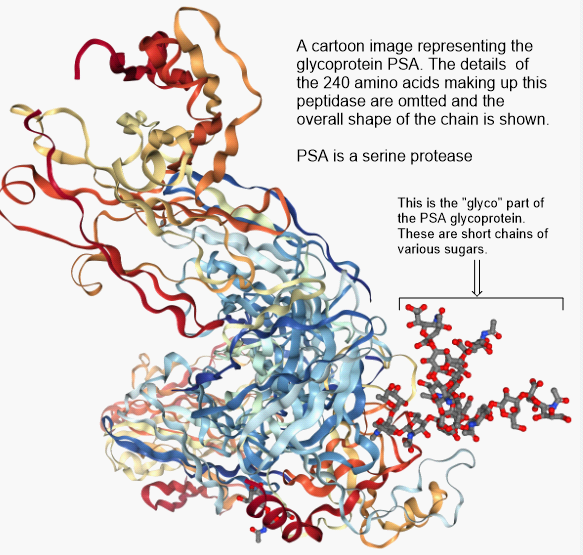
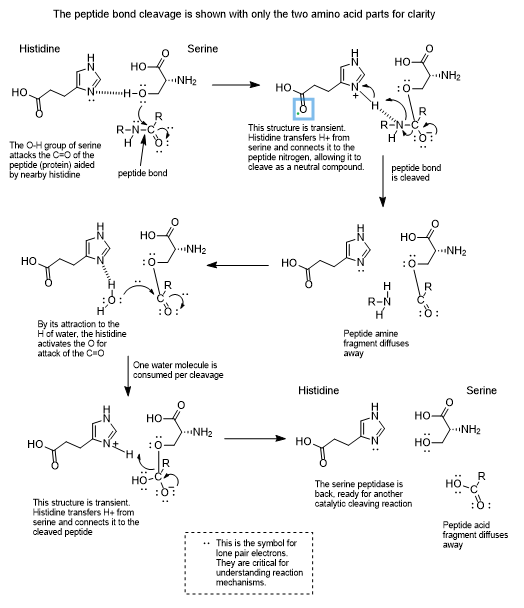
Serine protease enzymes like PSA have a serine amino acid in the active site of the enzyme which is capable of connecting temporarily with a carbonyl carbon of a (C=O) peptide bond. Since proteins are long chains of peptide bonds, cleaving a peptide bond snips the protein into smaller pieces.
Chemists are all about the mechanisms of chemical transformations and the following has been proposed for a serine protease.
All this said, it turns out that when castration resistance sets in, things begin take a turn for the worse. Prostate cancer cells begin to accumulate in the bone marrow, they begin to interact and develop into tumors that are essentially beyond the reach of treatment. The spine is a common place for them to go, but they can spread to other organs as well.
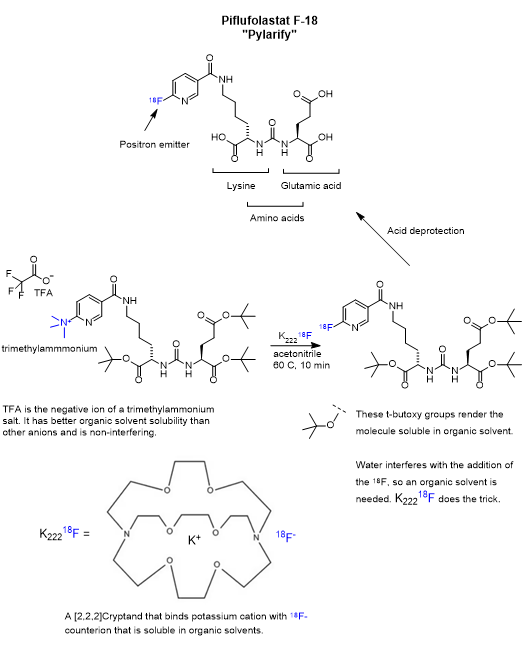
Of particular interest is the spread of prostate cancer to bone. Prostate cancer cells have an affinity for bone marrow tissue. In my case, the PET/CT scans gave no indication of being present in the head & neck, the chest or bones. That’s good news. In my first round of treatment, I was given 18F-Glucose diagnostic for the PET scan. This time I was given the more receptor-selective 18F PSMA diagnostic called Pylarify. While it is selective for a particular receptor on the cancer cell, it also shows up elsewhere in the body in the PET scan as a result of circulation and transport out of the system. Receptor-specific drugs will bind to the intended receptor, but only after they wander around and stumble into it. This is made less than random due to active transport or solubility partitioning. The effectiveness also benefits by resistance to metabolism and excretion.
Pylarify is a kind of pseudo-peptide containing two modified amino acids, lysine and glutamic acid, joined at the nitrogen atoms as a urea linkage. The key step is the nucleophilic aromatic substitution of trimethylammonium by 18F on the pyridine ring. The presence of abundant heteroatoms (nitrogen and oxygen) groups is not uncommon for pharmaceuticals and is absolutely ordinary for proteins. Heteroatoms serve as hydrogen bond donors and acceptors which is critical in biochemical transformations. A hydrogen bond donor can reversibly bind to a hydrogen bond acceptor and keep the molecules in close proximity long enough for a transformation as well as participate in it.
Pages 29-51, ISSN 0969-8051, https://doi.org/10.1016/j.nucmedbio.2021.12.005.
The interaction of prostate cancer cells in the bone marrow environment is fairly complex and is well described by Zhang X. Interactions between cancer cells and bone microenvironment promote bone metastasis in prostate cancer. Cancer Commun (Lond). 2019 Nov 21;39(1):76. doi: 10.1186/s40880-019-0425-1. PMID: 31753020; PMCID: PMC6873445.
The interaction of prostate cancer cells with bone marrow cells is a topic for another day.