My, my, my. Rober F. Kennedy Jr. really screwed the pooch with his comments on ethnically targeted COVID-19. Reportedly, he said “there is an argument that (COVID-19) is ethnically targeted”, adding “Covid-19 is targeted to attack Caucasians and Black people. The people who are most immune are Ashkenazi Jews and Chinese …. we don’t know whether it’s deliberately targeted or not.” If this quote is correct, he did not actually say that COVID-19 was ethnically targeted, but rather that “there is an argument …”. It is much like saying “is Bob still beating his wife? I just don’t know …” Whether he endorses the targeting theory or not isn’t clear, but he was willing to trot out this provocative statement to make his point. There was much blowback. Given the racial undertones, it was a large blunder.
RFK Jr. is well known as an advocate for conspiracy theories, some of which are whoppers. The online news magazine Slate has an article that compiles them. I find that his portfolio of mania is exhausting. The thought of pushing back against such seems like a fool’s errand. It reminds of a line in the movie True Grit: “What have you done when you have bested a fool?” What is the point in debating him?
RFK Jr. is a Harvard grad and went the University of Virginia School of Law to get his JD degree. He had a few slip ups early in his career but recovered. He spent most of his career as an environmental lawyer and has fought many laudable battles for environmental justice. Somewhere along the line he went off the rails and landed in the crackpot ferry to conspiracy land. RFK Jr. is a penetrating anti-vaccine voice who can draw large crowds if for no other reason just to see him.
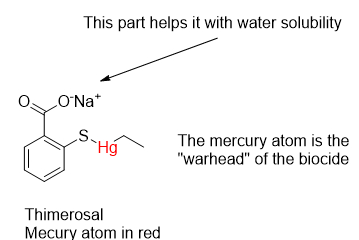
The substance of concern behind much of the anti-vaccine Sturm und Drang is Thimerosal. It is a synthetic organomercurial compound that is effective against bacteria and fungi. Its biocidal properties have been known since the around 1930. Mercurials have been used since the time of the Swiss alchemist Paracelsus (Philippus Aureolus Theophrastus Bombastus von Hohenheim) in the 1500’s. Paracelsus is known for the pronouncement that “only the dose makes the poison.” This remains a fundamental principle of toxicology.
The early mercurial medicaments used by Paracelsus were simple inorganic salts of mercury(II) like mercuric chloride, HgCl2, or mercury(I) like mercurous chloride, Hg2Cl2, also known as the mineral calomel. Mercuric chloride is prepared by treating liquid mercury with sulfuric acid followed by addition of sodium chloride for anion exchange. Mercurous chloride is prepared by heating mercuric chloride with mercury to do the reduction of Hg++ to Hg+.
Thimerosal is sometimes wrongly compared to methylmercury, a known and tragically toxic compound with the formula CH3Hg+X–. The X anion can be chloride, hydroxide or a thiol, depending on the source. It is an easy comparison to make because of the similarity of methyl (CH3) to the ethyl (CH3CH2) hydrocarbon group in Thimerosal, but research has proven it to be a poor comparison. Methylmercury compounds can be produced by aquatic microorganisms in water bodies in the presence of inorganic mercury. The methylation of natural biomolecules is a well-known process.
Like many metals, mercury has an affinity for sulfur, occurring naturally as mercury (II) sulfide, HgS, as deposits of Cinnabar or as a minor constituent with other minerals. It also has an affinity for sulfur-containing amino acids such as methionine, cysteine and homocysteine found in proteins. In the bloodstream mercury binds with proteins like albumin to the extent of 95-99 %. While in the body and exposed to water it decomposes to thiosalicylate and ethylmercury. Ethylmercury cation (CH3CH2Hg+) disperses widely and can cross the blood-brain and placental barriers.

According to Doria, Farina, and Rocha (2015) in Applied Toxicology, a comparison of effects between methylmercury and ethylmercury gave essentially the same outcomes in vitro for cardiovascular, neural and immune cells. Under in vivo conditions, however, there was evidence of different toxicokinetic profiles. Ethylmercury showed a shorter half-life, compartmental distribution and elimination compared to methylmercury. Methylmercury and ethylmercury toxicity profiles show different exposure and toxicity risks.
For many years, Thimerosal was sold as an antiseptic under the name Merthiolate as a tincture (an ethanol solution) by Eli Lilly and Co. Like most households in the 1960’s, we had it in the medicine cabinet or its cousin Mercurochrome. They were used for topical application to burns, cuts and scratches. Thimerosal has been used as a preservative in many health-related preparations such as vaccines, eye drops and contact lens disinfecting solutions. While the CDC has cleared it of doing harm, anti-vaccine mania hit the fan well before COVID-19 and RFK Jr. put his credibility and name recognition behind it.
Thimerosal was first prepared by chemist Morris Kharasch at the University of Maryland in 1927. An interesting technical summary of the substance can be found on Drugbank Online.

Kharasch is known for his pioneering work in free radical chemistry in the 1930’s at the University of Chicago but before that began his work with organomercury chemistry during the 1920’s while at the University of Maryland. His development of Thimerosal was a result of his organomercury work. He is also credited with opening the door to organic free radical chemistry leading to improvements in rubber polymer chemistry and manufacture. His work led to the use of peroxides to reliably induce the so-called anti-Markovnikov addition of a protic acid (HX) to olefins. The presence of trace peroxides was behind the unexpected “reverse” Markovnikov addition of seen in work with the addition of hydrogen bromide to bromopropene.
Kharasch’s early work in organomercury chemistry led to the invention (and patenting) of what became known as Merthiolate (thimerosal). Kharasch later worked as a successful consultant for Eli Lilly, the Du Pont Company, US Rubber, the US Army and others. In many cases these companies were the assignees of the patents.
Little mention is made of Morris Kharasch as a prolific and wide-ranging inventor with, by my count, 117 US patents with him as the inventor. So, why did Kharasch bother to patent Thimerosal? Did he anticipate its biocidal and preservative properties?
Kharasch references make mention of a 1931 patent regarding Thimerosal. That patent is STABILIZED BACTERICIDE AND PROCESS OF STABILIZING IT, US 1862896, appln. filed August 22, 1931, assignee: no party disclosed. The patent claims a process for and claims of water-soluble solution compositions. Numerous additives include antioxidants, alkyl amines, ethanolamine and borax. Claim 19 is telling. It claims the composition of sodium ethyl mercurithiosalicylate (Thimerosal), monoethanolamine, borax as a buffer and enough sodium chloride to make the composition sufficiently isotonic with the body fluids. In this patent the Thimerosal composition itself is not claimed, but as a component of a stabilized water solution. Claim 14 claims a water solution composition of sodium ethyl mercurithiosalicylate and an antioxidant which tends to “inhibit the acquisition” (odd choice of words) of burning properties by the solution. This plus the claim of an isotonic composition strongly suggests anticipated medicinal applications.
STABILIZED ORGANO-MERCUR-SULFUR COMPOUNDS, US 2012820, appln. Feb 17, 1934, assignee: Eli Lilly and Company. Claims a stabilized solution of alkyl mercuric sulfur compounds in water with aliphatic 1,2-diamines. Also claims Ethylenediamine ethylmercurithiosalicylate composition. This is similar to the ‘896 patent but specified ethylenediamines.
As mentioned above, the biocidal nature of inorganic mercurials had been known for a long time. There was actually limited success in the treatment of syphilis. But they were long known for being very harsh on the patient and grew out of favor when better treatments came along.
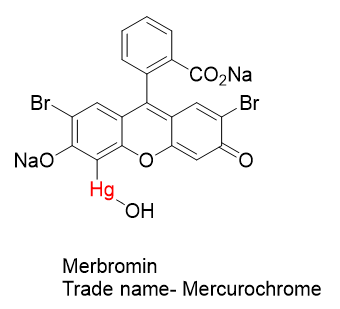
The antiseptic properties of Mercurochrome were discovered in 1918 at Johns Hopkins Hospital by urologist Hugh H. Young. Mercurochrome is essentially a dye molecule with an attached mercury warhead. There are three groups on the organic structure that aid in its solubility in water- NaO, CO2Na, and HgOH. Water solubility is often an important attribute in medicinal substances.
Given that antiseptic properties of organomercurials were known, it is perhaps not surprising that an enterprising Ukrainian immigrant with an interest in organomercurials like Morris Kharasch might want to patent his invention.