Just today, as the open door to my golden years stands gaping before me, I learned of a substance called dioxygen difluoride, FOOF. It’s also known as perfluoroperoxide at the NIST Chemistry WebBook site. Seems like I’m always the last one to the oxidizer party. This rather unhappy substance can be prepared as shown below. The word is that the orange-yellow solid is only stable below −160 °C.
O2 + F2 → O2F2 (electric discharge, 183 °C) Wikipedia.
2 O3F2 → O2 + 2 O2F2
Another synthesis can be found in a 1991 paper in the Journal of Fluorine Chemistry.
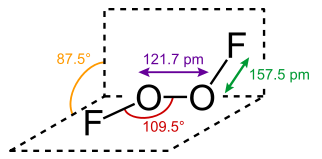
Image from Wikipedia. https://en.wikipedia.org/wiki/Dioxygen_difluoride. A mixture of fluorine and oxygen gas were heated to 700° C then, according to the abstract “rapidly cooled on the outer surface of stainless steel tubes. The tubes were refrigerated by a liquid oxygen bath pressurized to >7600 torr with helium. Six grams of O2F2 were produced in less than an hour.”
Derek Lowe mentioned in one post in his Blog In the Pipeline that FOOF was in the list of materials he won’t work with. Derek also mentioned chlorine trifluoride. A method of preparing this substance is shown below. This substance is a powerful fluorinating agent and reacts in hypergolic fashion with asbestos and sand according to chemist John Drury Clark. Clark wrote a book called Ignition! An Informal History of Liquid Rocket Propellants based on his experiences with rocket propellant research. Clark said that the great toxicity of ClF3 was the “least of its problems”. It’s ability to react in a hypergolic manner with nearly everything was a barrier to its use. It could be stored in metal containers that were first passivated with fluorine gas.
3 F2 + Cl2 → 2 ClF3
Uranium hexafluoride is produced with chloride trifluoride-
U + 3 ClF3 → UF6 + 3 ClF
According to Wikipedia, ClF3 is used to clean Chemical Vapor Deposition (CVD) chambers. Not surprisingly, prior to WWII the Nazis had experimented with ClF3 as a chemical warfare agent called N-Stoff. Production halted when the Red Army overran the facility in 1945. The substance was never used in war.

This post reminded me of when the late Ozark Fluorine Specialties company was faced with disposing of a quantity of tetrafluorohydrazine (N2F4) which had been sitting idly in storage for years. That was fun.
Ok, I missed this comment. How does one dispose of N2F4 and survive to tell the story? Pray, do tell.
Well, it’s somewhat of a story. At the time, Ozark was owned by Atochem and corporate health and safety somehow found out that the former Technical director at Ozark was a pack rat, collecting all kinds of chemicals that had fluorine in them. That included a few dozen cylinders of N2F4 he acquired from, I believe, Hercules Chemical. Leftover stock from a production run for Redstone Arsenal.
In any event, a quick search of the properties of N2F4 led them to immediately build a refrigerated room around the pallets of cylinders inside the warehouse in Tulsa housing the cylinders. It was a Sears shed with an AC unit plugged into the wall.
They also contacted several hazardous waste disposal companies and learned that getting rid of these cylinders would be an expensive proposition, upwards of 6-figures if memory serves me.
I was the Business Manager at the time and went looking for someone to take them off our hands. Karl Christie at USC and with the USAF Edwards Air Force Base at the time took an interest and I offered to sell him the entire lot for $1. We would even cover the cost of shipping to the destination of his choice.
It seemed that N2F4 was a possible reagent for certain high energy rocket propellants. I later learned that we were apparently sitting on the entire free world’s supply of N2F4.
We struck a deal and everybody was happy.
You are so damned lucky. John B. had a big and old cylinder of N2O4 laying on the ground that he had to pay to get rid of. The valves were frozen and couldn’t be opened or removed. Never heard where it went.