Found some YouTube videos on liquid chlorine releases by the Chlorine Institute. The first video is from the Dugway Proving Grounds and shows how a liquid chlorine release will proceed depending on the direction of the release. I won’t spoil it by yammering on about it.
Category Archives: Chemical Industry
Gaussling’s Epistle to the Bohemians 2/28/23
>>> A smattering of thoughts each too small for a post. <<<
I’ve been thinking about quantum chemistry lately, or more to the point, my graduate-level single semester experience with it. First let me say that prior to taking the qualifying exams on arrival to the graduate chemistry program, I made sure to bone up on the particle in a one-dimensional box model. And sure enough, it was on the entry p-chem exam. Whew! Dodged that bullet. However, of all 5 exams we took, I didn’t pass the statistical mechanics exam. I would have to repeat the exam and pass it by the end of the year. Instead of taking the undergrad p-chem course I decided to risk it and study on my own and as luck would have it, I managed to pass it. Another monkey off my back.
Back to the quantum chemistry course. Initially I was hoping to gain a bit of qualitative insight into the subject. As it turned out, it was really just a high level math class where the prof spent the whole term deriving all of the key equations. I think this is pretty common for this subject. There were zero interesting applications mentioned. He was either unable or unwilling to render any of it into sentences for context. The guy was a rock star in his area of solid state nuclear magnetic resonance. Once I went in for help during office hours and he told me he was busy and to come back in 2 weeks (!). I was finally convinced that putting scientists on a pedestal was a serious error and that a**holes were truly everywhere. Anyway, I made it through the experience and moved on. Haven’t had to think about Hamiltonians since.
==========
I was chatting with a toxicologist colleague recently about the big derailment and fire disaster in East Palestine, OH. I had suggested that the decision of the responders to vent and burn the remaining vinyl chloride was probably a good idea. There was some fear that there may be a runaway polymerization of the vinyl chloride. This would likely lead to an explosive rupture of the tank car and a possible BLEVE. This is from the report–
“On February 5, responders mitigated the fire, but five derailed DOT-105 specification tank cars (railcars 28–31 and 55) carrying 115,580 gallons of vinyl chloride continued to concern authorities because the temperature inside one tank car was still rising. This increase in temperature suggested that the vinyl chloride was undergoing a polymerization reaction, which could pose an explosion hazard. Responders scheduled a controlled venting of the five vinyl chloride tank cars to release and burn the vinyl chloride, expanded the evacuation zone to a 1-mile by 2- mile area, and dug ditches to contain released vinyl chloride liquid while it vaporized and burned. The controlled venting began about 4:40 p.m. on February 6 and continued for several hours.”
My colleague said that a fire releases aerosols that are likely to be especially deleterious to the lungs. Burning organic chlorides leads to hydrochloric acid formation with all of the joy that it brings to the dance. The smoke plume, elevated by convection, and probably carrying some amount of unburned chemicals will spread with the aerosols far and wide. This would contaminate a larger patch of environment and expose a more distant population than a simple spill at the crash site would. He wondered to what extent the chemicals shouldn’t have been removed at the site, spill or not, and the land be designated as a Brownfield.
==========
Elon Musk has been running off at the mouth again, this time seeming to take sides with the Dilbert cartoonist Scott Adams who was recently given the death penalty of abandonment by his publishers. Adams used his cartoon to go off on the Black population saying that Whites “should get the hell away from Black people” referring to them as a racist hate group.
Set aside the merits/demerits and morality of Adams’ racial views for a minute. As an adult and businessman he should have known the boundaries of acceptable content in his cartoon strips in the current social environment. He published content that appeared to have alignment with white supremacist ideas. In publishing this content, he made himself radioactive and he was dropped by his publishers who happen to have better business sense. What a dunce. He was playing with a loaded gun and it went off in his face.
So, His Excellency, Elon Musk, has stepped into the fray and condemned the excommunication of Adams from the comic strip pages. Musk said that while Adams’ comments weren’t good, there was an element of truth in them. He accused the media of providing a “false narrative” by giving more attention to Black victims of police violence than to White victims of police violence. This is on top of his general loosening on hate speech on Twitter and the reinstatement of banned accounts such as with #45. Musk is broadcasting that hate speech is as valid as any other speech on his platform. Businesses like Twitter are free to edit content or not as they please. Musk believes in a rough-and-tumble environment where most anything goes. As an owner, he is certainly free to do that. But as owner, he is also responsible for content that drives away business.
Irrespective of your beliefs in this matter or the obvious morality issues, it should be apparent that neither Adams or Musk seem to care about the effect on business of draping yourself in the flag of racism, or even just of allowing the perception of it. Savvy is a kind of vector- it has magnitude and direction. Musk has strong vectors in the technology direction, but not so much in the public relations direction. He doesn’t seem to have full control of his mouth just yet.
==========
The burnin’ ring of fire
The Norfolk Southern train derailment and fire in East Palestine, OH, has spread into the political dumpster. By not appearing near the crash site promptly, both Biden and Buttigieg are feeling the heat of the GOP panic machine. The single plank on the GOP platform is to knock down Democrats at every opportunity. While the news organs of the GOP are busy trying to blame the Biden administration for the accident, fire and contamination, citizens are expressing dismay over not knowing what to do going forward. They aren’t receiving much advice or direction from EPA about how much they should be worried about contamination and exposure to the released chemicals. In fact, on the ground it has been hard to see the hand of government anywhere. Their frustration is normal and understandable. I would be frustrated too.
Let’s step back a minute and examine the situation from 50,000 ft. The last thing we want in government is for a proper response to an emergency of this scale to require the president to personally lead the emergency response. The same is true for the Secretary of Transportation. Good leaders delegate responsibility to specialists for situations like this. Good leaders are watchful but stay out of the way of the experts. Good leaders make sure that the people on the ground have the resources they need to do their jobs. Ok, Biden didn’t respond publicly to the situation early enough, but that is not to say that things weren’t happening. But, he has 330,000,000 American back-seat drivers to make happy. That’s his job.
Let’s remind ourselves that Biden and NATO are also busy trying to prevent the start of WWIII.
As with an emergency of any scale, it takes responders some time to understand the situation and then to bring resources to bear. In the mean time, the NTSB was promptly dispatched and has already published preliminary report RRD23MR005 on the event. It is very interesting to see that many of the safety systems worked. The overheated wheel was detected and an emergency braking procedure was put into action just before the derailment occurred.
Ok, Biden and Buttigieg could have been quicker to publicly extend sympathy and the promise of relief. Complaining about this is like accusing grandma of not giving you a kiss while she was trying to put out a fire in the kitchen. But contrast this PR error of omission with the antics of #45 in Puerto Rico after the recent hurricane. Remember how he tossed rolls of paper towels as mock support during an interview down there? MAGA people have no leg to stand on with presidential expressions of sympathy.
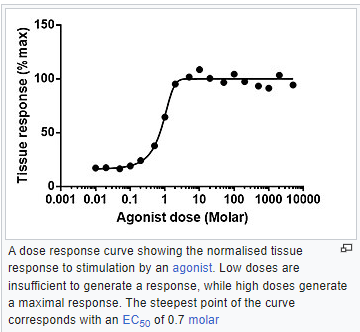
As far as what kind of toxic threat there is to humans and what potential environmental insult there will be, the situation has not fully played out yet. This will need to be studied for years. There is acute toxicity and there is chronic toxicity. With most chemicals there will be a clear dose-response relationship with chemical dosing if you choose the right experiment to do. However, that relationship can become quite uncertain with low dosing. The health effects of exposures from the East Palestine derailment cannot be measured with high precision over the long haul. Genuine toxic effects are over-printed on a background of natural disease. Diseased tissues do not have little signs that say “I was caused by vinyl chloride dosing”. Histology can characterize cell types and correlate them with known chemical insult, but only a jury can say if any particular conclusion will hold up in court. With toxicity effects, certainty is not always what you get.
Paracelsus said in 1538 that “All things are poison, and nothing is without poison; the dosage alone makes it so a thing is not a poison“. That observation is still true today.
Hazardous Metaphor On Fire in Ohio
When I think “train wreck” I usually think of #45’s presidency. But here I refer to the actual Feb 3, 2023, Norfolk Southern train wreck in East Palestine, OH. A very long train carrying, among other things, tankers of hazardous chemicals had a derailment and fire near the small town of East Palestine, OH, along the southwest Pennsylvania border. It was a true calamity releasing hazardous chemicals, some of which caught fire and burned for days. It isn’t clear as yet as to what burned and what didn’t. The extent of pollution will eventually be released by authorities and monitored for years to come.
Early reports have claimed that the accident started with an overheated wheel bearing. It would be interesting learn how this could lead to a derailment. The root cause analysis will be interesting.
According to Wikipedia–
NTSB chair Jennifer Homendy explained that the train in this accident would not have been required to utilize the ECP braking system even if the FAST Act was not repealed, because the term high-hazard flammable train means a single train transporting 20 or more tank cars loaded with a Class 3 flammable liquid. As it had only three such placarded train cars, the derailed train did not meet the qualifications of a “high-hazard flammable” train.
ECP stands for Electronically Controlled Pneumatic brakes. The Wikipedia page describes the pathetic political kerfuffle over these brakes and how certain groups fought the requirements for them.

The Washington Post released a piece, dated Feb 18, 2023, about it showing some interesting pictures. One aerial shot captures the wreckage along with what the cars were carrying. A security camera caught the train moving along with a large fire blazing under one car minutes before entering town. The video has since been removed.
The burning vinyl chloride (and … ?) produced a toxic plume that by some accounts was also corrosive. I assume this to be due to the burning of an organic chloride releasing hydrochloric acid vapors. According to Wikipedia, of the 150 cars in the train some 38 train cars were derailed.
Substances in cars that were derailed according to the Washington Post-
- Vinyl chloride
- Polyethylene
- Dipropylene and propylene alcohol
- Semolina (a wheat flour)
- Polyvinyl chloride (PVC)
- Ethylhexyl acrylate
- Petroleum lubricating oil
- Diethylene glycol
- Isobutylene
- Butyl acrylate
- Benzene
Much was made in the news about burning vinyl chloride and noxious fumes, but I haven’t heard an accounting of what actually burned. Any release of acrylate monomers is especially unfortunate since as a group, they can be nasty lachrymators. This will take years to get through the courts.
New Ethane Cracker for Europe
INEOS in planning to put a new ethane cracker in the ground in Antwerp, Belgium, called Project One. INEOS has reportedly raised 3.5 billion Euros for the construction. The new plant will have a carbon footprint 3 times lower than the average European steam cracker. The process will use so-called “low carbon hydrogen” to power the cracker. A cursory search of Google didn’t produce a clear definition of low carbon hydrogen. Maybe the reader has an idea. The hydrogen literature has gotten quite complicated with the large variety of hydrogen sources and technologies.
An ethane cracker removes one molecule of hydrogen from each molecule of ethane to produce one molecule of ethylene product. Of course, ethylene is the primary monomer for all of the various grades of polyethylene (a polyolefin). This uptick in capacity is likely driven by optimistic projections for increasing demand for polyolefins. Alternatively, it could be in anticipation of retiring capacity.

Other feedstocks like LPG or naphtha can be cracked to produce a different spread of unsaturated and aromatic products. Olefins produced feed into a variety of large-scale manufacturing streams.
In a cracker the ethane is diluted with steam and briefly heated to ca 850 C for a few milliseconds and then quickly quenched. Steam crackers are constructed to capture waste heat from the process to power refrigeration compressors. Production of ethylene is very energy and carbon intensive. According to Wikipedia, for every 1 tonne (1000 kg) of ethylene there are 1 to 1.6 tonnes of carbon dioxide produced, depending on the feedstock. This plant is designed to reduce carbon output.

Filthy Lucre, Again
Yet another reprint of posts from the past, this time from April 11, 2008.
As usual, Th’ Gaussling’s most interesting observations of the ACS meeting are of a proprietary nature and will have to go with me to the grave. Our student and academician friends can expound openly on what lights their fires. The lusty satisfaction of compelling oratory in the darkened halls of convention centers is part of the reward for the cardinals of the academy. Members of the merchant class have to be satisfied with better dining.
People who are involved in personnel issues often speak of an employees “deliverables” as their work product. For those lucky enough to be in the academy, the work product includes teaching young minds, conducting research, and participating in the dissemination of the results in the form of papers and conferences.
For we chemists who did the deal with the devil in exchange for filthy lucre, our performance is rated somewhat differently. Like academics, our performance metric only starts with some understanding of science. Once it is possible to begin understanding a thing, the task of transforming a process or material property into an item of commerce begins. In the chemical industry we do the most important reaction of all- the transformation of chemicals into money.
The part of the brain that sees a stick on the forest floor that resembles a tool is the same part of the brain that scans a molecule and sees latent functionality or value. The extraction of value from a composition or a process is a complex anthropological activity. Product development is anthropological because it involves the use of tools and organizational structure to provide products or services that are exchanged between groups.
An industrial science group has to isolate value in some material property and contrive to bring some product or service into being. But to get it to market, the science tribe has to cooperate with those with other skills. Organizations often resemble a confederation of tribes who cooperate with complex rituals and methods of exchange.
US LNG output to double by 2027
According to BloombergNEF, the United States is on course to double its natural gas liquefaction (LNG) capacity by 2027. US export capacity is expected to rise to 169 million metric tons per year with the opening of 3 new projects slated for funding approval this year. They are- Venture Global’s Plaquemines LNG, Sempra’s Port Arthur LNG, and NextDecade’s Rio Grande LNG. This new capacity will place the US well ahead of Qatar in annual production.
Appendix–
LNG should not be confused with LPG, Liquified Petroleum Gas. LPG is a mixture of the somewhat heavier hydrocarbons propane, propylene, butylene, isobutane and n-butane. LPG is a fuel gas and can be used as an aerosol propellant and refrigerant.
LNG is composed mainly of methane (CH4) with a smaller amount of ethane (C2H6). Lesser amounts of propane and butane are isolated and sent to a separate stream. Natural gas is “sweetened” prior to cooling to remove corrosive hydrogen sulfide (H2S), carbon dioxide (CO2) gases as well as helium, mud, water, oil and mercury. Once the impurities are removed, the remaining methane/ethane mixture is cooled to −162 °C for bulk transport. On arrival at its destination, it must undergo a regasification process. In some locations seawater can be used to vaporize the LNG for injection into pipelines.
As an alternative to sea water heat transfer for regasification, LNG can be utilized for its “cold energy” potential. One application uses low temperature LNG as a refrigeration coolant for producing liquid oxygen and liquid nitrogen. Another use of the cold energy is to cool the exhaust of a gas turbine in a closed joule cycle with argon as the fluid.
Since we are talking about gaseous hydrocarbons, there is also a category of liquid hydrocarbons called condensates that accompany the production of natural gas and must be channeled into a separate processing stream because, well, they are liquid. Raw natural gas straight out of the ground may have varying amounts of condensates-
- Crude oil wells can produce natural gas called associated gas and condensates may be entrained in the gas flow.
- Dry gas wells produce gas that have no associated liquids.
- Condensate wells produce natural gas with associate natural gas liquid.
Wikipedia explains the condensate situation in greater detail.
On the Release of Hazardous Energy for Chemists
This post is an update of a post I wrote on Mole-Day of 2011. It is a brain dump that summarizes much of what I’ve learned about dealing with potentially explosive chemicals in the manufacturing environment. Very few chemists actually have to deal with explosive chemicals in their work activities. It is actually quite uncommon. No doubt some important considerations have been left out and for that I apologize.
The Prime Directive: If you choose to bring in or make a chemical substance in your facility, you must develop in-house expertise in the safe handling and use of that substance. Do not expect to rely on outside expertise for it’s safe use. Always strive to build in-house expertise in regard to chemical properties and safety- never farm this out to consultants. This includes proper engineering and broad knowledge of reactive chemical hazards.
Safety has a substantial psychological component. You can build into a chemical manufacturing process extensive engineering and administrative controls for safe operation. These layers of control are concrete and definable. What is fuzzy, however, is the matter of how people behave. In particular, I’m thinking of getting people to behave in a particular way over the long haul. Keeping people operating safely over long periods of time where no adverse events happen poses special problems. Especially in regard to low frequency, high consequence events. Cutting corners and improper use of PPE is not uncommon and should be expected. Something expected can be watched for continuously.
In safety training I mention that handling a hazardous material is like handling a rattle snake. You have to exercise the due caution every single time you pick up that snake. You do not accumulate and bank safety credits for previous safe handling. Everybody understands this already at some level. But the possibility of drift in safety practice over time needs to be emphasized.
The best strategy I know of besides complete process automation is recurrent safety training along with vigilant management. Successful safety management requires proper supervision by alert supervisors. Management by walking around helps with this. Well written process instructions that anticipate practical problems are essential. Holding people accountable for following Standard Operating Procedures is critical. Working conditions conducive to focus are always good. Operational rotation with may be helpful.
In chemical safety, the biggest worry is typically the potential for an explosion. What should you do if a raw material or product in a process may be explosive or has explosive features on the molecule? Good question. First, someone in the R&D chain of command should have knowledge of the list of known explosophores. It’s not a big list. PhD chemists in R&D should know this anyway. Explosive molecules have certain chemical bonds that are weakest and are known as “trigger bonds“. It is thought that the rupture of these trigger bonds initiates explosive decomposition of the substance.
Just because a material has explosive properties does not automatically disqualify it for use. Azides and nitro compounds are used safely every day. But, to use a chemical safely you must accumulate some knowledge on the type and magnitude of stimulus that is required to give a hazardous release of energy.
For any given hazard, it is my personal policy to learn as much about the nature of the hazard at the chemical and bulk level as I can. I believe that it is important to know more about something than what is immediately called for. That is the difference between education and training. This is how you build expertise.
Some comments on the release of hazardous energy. Hazardous energy is that energy which, if released in an uncontrolled way, can result in harm to people or equipment. This energy may be stored in a compressed spring, a tank of compressed gas, the stable chemical bonds of a flammable material, the unstable chemical bonds of an explosive material, or as an explosive mixture of air and fuel. A good old fashioned pool fire is a release of hazardous energy as well. Radiant energy heating from a pool fire can easily and rapidly accelerate nearby materials past the ignition point. Good housekeeping goes a long way towards preventing the spread of fires.
Applying and accumulating energy in large quantities is common and actually necessary in many process activities. In chemical processing, heat energy may be applied to chemical reactions. Commonly, heat is released from chemical reactions at some level ranging from minimal to large. The rate of heat evolution in common chemical reactions can be simply and reliably managed by controlling the temperature or rate of addition of reactants where two reactants are necessary. However, reactions do not always evolve significant power output immediately on mixing of the reactants.
Induction periods are potentially dangerous and must be identified prior to scale up. The appearance of an exotherm very early in a feed operation is a good indication that the reaction has begun. However, a thermogram from a reaction calorimeter showing the temperature and power output (watts) versus the feed mass will indicate if the reaction is slow and accumulation of reagent (energy) is occurring. This can be teased out early by adding a small shot of reactant feed (a few %) and watching the power profile. The ideal profile is where the power output starts promptly, peaks and then promptly decays to baseline. This is a good indicator of the absence of accumulation. Generally, the kinetics are most favorable at the beginning of the reagent feed and taper off to zero as reactants are consumed. Some accumulation is usually tolerable from the heat load perspective. This is a good thing because a thermogram showing some accumulation could lead to an unnecessarily long feed time. A reaction calorimeter can give the peak wattage per kilogram of reaction mass. An engineer should be able to estimate the maximum controllable heat flux for a given reactor. Without being too specific, it is in the range of several tens of watts per kg of reaction mass according to one reference I know.
There are explosive materials and there are explosive conditions. If one places the components of the fire triangle into a confined space, what may have been simple flammability in open air is now the makings of an explosion. Explosive materials have two legs of the fire triangle built into the molecule- the oxidizer and the fuel separated by only nanometers. However, the composition of the explosive itself may not produce a balanced reduction/oxidation reaction. The oxygen balance is a easily calculated number that will indicate whether or not there is an excess or deficit of oxygen in an explosive substance. For example, ammonium nitrate has a 20 % excess of oxygen. Fuel oil can be added to bring the fuel/oxidizer ratio into redox balance. This mixture is referred to as ANFO.
In a chemical explosion, heat and increasing pressure can do PV work on the contents and containment. Minimally, the outcome will be an overpressure with perhaps the blowing of a rupture disk on a reactor. In another situation, the equipment may blow apart and send fragments flying away at high speed with an expanding fireball.
There is a particular type of explosive behavior called detonation. Detonation is a variety of explosive behavior that is characterized by the generation and propagation of a high velocity shock through a material. A shock is a high velocity compression wave which begins at the point of initiation and propagates throughout the bulk mass of explosive material. Interestingly, because it is a wave, it can be manipulated somewhat by reflection and refraction. This is the basis for explosive lensing and shaped charges. It is characteristic of detonations to produce shredded metal components. Detonations have a very large rate of pressure rise, dP/dt. The magnitude of dust explosions is commonly performed by a few commercial test labs out there. One of the important test results is the Kst value showing the magnitude of the explosive force.
Detonable materials may be subject to geometry constraints that limit the propagation of the shock. A cylinder of explosive material may or may not propagate a detonation wave depending on the diameter. Some materials are relatively insensitive to the shape and thickness. A film of nitroglycerin will easily propagate as will a slender filling of PETN in detonation cord. But these compounds are for munitions makers, not custom or fine chemical manufacturers. The point is that explosability and detonability is rather more complex than one might realize. Therefore, it is important to do a variety of tests on a material suspected of explosability. The type and magnitude of stimulus necessary to produce an explosion must be understood for safe handling and shipping.
A characteristic of detonable explosives is the ability to propagate a shock through the bulk of the explosive material. However, this ability may depend upon the geometry of the material, the shock velocity, and the purity of the explosive itself. There are other parameters as well. Marginally detonable materials may lose critical energy if the shape of the charge provides enough surface area for loss of energy.
Explosive substances have functional groups that are the locus of their explosibility. A functional group related to the initiation of explosive behavior, called an explosophore, is needed to give a molecule explosability. Obvious explosophores include azide, nitro, nitroesters, nitrate salts, perchlorates, fulminates, diazo compounds, peroxides, picrates and styphnates, and certain hydrazine moieties. Other explosophores include the hydroxylamino group. HOBt, a triazole analog of hydroxyamine, hydroxybenzotriazole, has injured people, destroyed reactors and caused serious damage to facilities. Anhydrous hydroxylamine has been the source of a few plant explosions as well. It is possible to run a process for years and never cross the line to runaway as was the case for these substances.
Let’s go back to the original question of this essay. What do you do if you find that a raw material or a product is explosive? The first thing to do is collect all available information on the properties of the substance. In a business organization, upper management must be engaged immediately since the handling of such materials involves the assumption of risk profiles beyond that expected.
At this point, an evaluation must be made in relation to the value of the product in your business model vs the magnitude of the risk. Dow’s Fire and Explosion Index is one place to start. This methodology attempts to quantify and weight the risks of a particular scenario. A range of numbers are possible and a ranking of risk magnitude can be obtained therein. It is then possible to compare the risk ranking to a risk policy schedule generated beforehand by management. The intent is to quantify the risk against a scale already settled upon for easier decision making. A problem with this approach is that it requires numerical values for risk which might be difficult to come by.
But even before such a risk ranking can be made, it is necessary to understand the type and magnitude of stimulus needed to elicit a release of hazardous energy. A good place to start is with a DSC thermogram and a TGA profile. These are easy and relatively inexpensive. A DSC thermogram will indicate onset temperature at a given temperature ramp rate and energy release data as a first pass. Low onset temperature and high energy release is least desirable. High onset temperature and/or low exothermicity is most desirable.
What is more difficult to come to a decision point on is the scenario where there is relatively high temperature onset and high exothermicity. Inevitably, the argument will be made that operating temperatures will be far below the onset temp and that a hazardous condition may be avoided by simply putting controls on processing temperatures. While there is some value to this, here is where we find that simple DSC data alone may be inadequate for validating safe operating conditions.
Onset temperatures are not inherent physical properties. Onset temperatures are kinetic epiphenomena that are dependent on the sensitivity of the instrument, sample quality, the Cp of both the sample and the crucible, and the rate of temperature rise. What may be needed once an indication of high energy release is indicated by the DSC is a determination of time to maximum rate (TMS). While this can be done with special techniques in the DSC (i.e., AKTS), TMR data may be calculated from 4 DSC scans at different rates, or it may be determined from Accelerated Rate Calorimetry, or ARC testing. Arc testing gives time, temp, and pressure profiles that DSC cannot give. ARC also gives an indication of non-classical liquid/vapour behavior that is useful. ARC testing can indicate the generation of non-condensable gases in the decomposition profile which is good to know.
Time to maximum rate is measured in time at a specified temperature. Many people consider that a TMR of 24 hours at the process temperature is a minimum threshold for operational safety. Others might advise 24 hours 50 or 100 C above the maximum operational temperature. If you contemplate using this parameter, it is critical to get testing from a professional lab for a time at a particular temperature. This kind of test will produce a formula that you can calculate TMR values at a given temperature. Bear in mind, however, that no outside safety consultant will tell you what you must do for liability reasons. You must develop enough in-house expertise to make this decision for yourself.
The standard tiered test protocol for DOT classification is a good place to start for acquiring data on explosive properties. Several companies do this testing and give ratings. There are levels of testing applied based on the result of what the lower series tests show. Series 1 and 2 are minimally what can be done to flesh out the effects of basic stimuli. What you get from the results of Series 1, 2, and 3 are a general indication of explosibilty and detonability, as well as sensitivity to impact and friction. In addition, tests for sensitivity to electric discharge and dust explosion parameters should be performed as well.
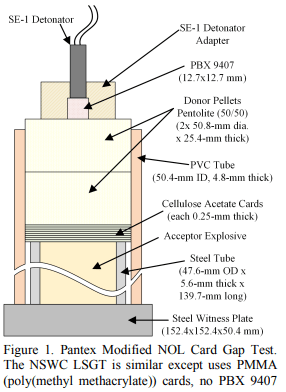
The card gap test, Konen test, and time-pressure test will give a good picture of explosive behavior. The Konen test indicates whether or not extreme heating can cause an explosion sufficient to fragment a container with a small hole in it.
BOM or BAM impact testing will indicate sensitivity to impact stimulus. Friction testing gives threshold data for friction sensitivity.
ESD sensitivity testing gives threshold data for visible effects of static discharge on the test material. Positive results include discoloration, smoking, flame, explosive report, etc.
Once the data is in hand, it is necessary to sift through it and make some business decisions. There is rarely a clear line on the ground to indicate what to do unless there is already a policy on decision making here. What testing results will indicate is what kind of stimulus is necessary to give a positive result with a particular test. It is up to your in-house experts and management to decide the likelihood of exposing the material to a particular stimulus. Will it be possible to engineer away the risk or diminish it to an acceptable level? The real question for the company is whether or not the risk of processing with the material is worth the reward. Everyone will have an opinion.
The key activity is to consider where in the process an unsafe stimulus may be applied to the material. If it is thermally sensitive in the range of heating utilities, then layers of protection guarding against overheating must be put in place. Layers of protection should include multiple engineering and administrative layers. Every layer is like a piece of Swiss cheese. The idea is to prevent the holes in the cheese from aligning.
If the material is impact or friction sensitive, then measures to guard against these stimuli must be put in place. For solids handling, this can be problematic. It might be that preparing the material as a solution is needed for minimum solids handling.
If the material is detonable, then all forms of stimulus must be guarded against unless you have specific knowledge that indicates otherwise. Furthermore, a safety study on storage should be performed. Segregation of explosable or detonable materials in storage will work towards decoupling of energy transfer during an incident. By segregating such materials, it is possible to minimize the adverse effects of fire and explosion to the rest of the facility.
With explosive materials, electrostatic safety is very important. All handling of explosable solids should involve provisions for the suppression of electrostatic charge generation and accumulation. A discharge of static energy in bulk solid material is a good way to initiate runaway decomposition of an energetic material. Unfortunately, some explosive substances may not require the oxygen leg of the fire triangle so, in this case, inerting with nitrogen won’t be preventative.
Safe practices involving energetic materials require an understanding the cause and effect of stimulus on the materials themselves. This is of necessity a data and knowledge driven activity. Handwaving arguments should also be suppressed in favor of data-driven analysis.
Oil Tanker Shipments
The Energy Information Administration (EIA) is a primary source of data relating to global petroleum and distillate use. It follows production, transport and prices. In addition to supplying data, they provide some interpretation of the global picture. There is so much BS circulating about fuel costs that a credible source of information is welcome.
Oil tankers come in two varieties- clean and dirty. A clean tanker hauls low-sulfur distillates. A dirty tanker hauls crude oil. Since the invasion of Ukraine, tanker shipments from Russia to the west have fallen off and longer voyage shipments have increased. This has increased the cost of transport and floating storage of petroleum and distillates. In the time between February 2022 and November 2022, Very Large Crude Carrier (VLCC) rates from the Middle East to the US Gulf Coast (USGC) have more than tripled. The rates from USGC to Rotterdam have increased from $8.00 to more than $27.00 per metric ton. Rates of shipments on Suezmax ships have also tripled. Dirty tanker rates from Russian ports in the Baltic and Black Sea have gone up due to increased insurance rates. Also, add to all of this the increased cost of bunker fuel for longer voyages.
Shipments of LPG (propane) have been delayed by long waiting times for passage through the Panama Canal. Congestion at the Neopanamax locks has led to increased scarcity of Very Large Gas Carriers (VLGC). Propane is both a fuel and an industrial feedstock. Propane is dehydrogenated to propylene and used for the production of polypropylene. Propane is also a fuel whose demand is highly seasonal with greatest demand in the winter months. VLGCs in the Middle East are drawn out of the area by better rates in the US, creating scarcity there.
Learn the Pragmatics of Perchloric Acid Chemistry Prior to Use
Forward
This article amounts to a plea to analytical chemists, supervisors, and organizations who use perchloric acid to make the effort to understand its reaction chemistry, as an acid or salt, and the peculiarities of the numerous mixtures used in analytical sample digestion. If your organization uses standard methods of digestion via one of the many acid mixtures and temperatures, it behooves your organization to have at least one individual on site who understands a bit more than just the procedure. If there is an incident of some kind involving perchloric acid, be it a spill, splash, or worse, having a grasp of the real hazard presented before you is useful. It is possible to underreact or overreact to any given incident scenario.
I am not an analyst. My interest is to understand reactive chemical hazards and devise means for preventing the transition from hazard to danger. Whether someone uses perchloric acid or not makes no difference to me. I have no investment in perchloric acid. However, I’m greatly interested in users being informed.
Comments on Safety Training
Safety training is commonly executed as a result of company policy where documentation of satisfactory completion is collected and filed. For lab chemists this includes training sessions on chemical storage, fire safety, fire extinguisher training, hazardous waste practices and regulations, storm water regulations, company safety and health SOP training, building evacuation, general lab safety, and perhaps basic first aid.
Often safety training sessions are canned professional video presentations or a corporate home brew of PowerPoint slide shows followed by some Q&A and a quiz. It is what I refer to as infotainment. Attendees may watch a video with dramatized incidents while the voiceover describes what should have happened. This approach is not without merit or some success, but this passive approach may not be of lasting value. Furthermore, it is a very sketchy assumption that such passive training will result in proper decision making in an off-normal circumstance where hazard may transition to danger.
The military has solved this problem long ago by mastering the art of the drill. They realize that if you need people to respond in a particular way rapidly, they have to be trained and drilled. In times of peace, the military has the opportunity to train and drill to maintain operational readiness. This is one way to address the difficult problem of low probability, high consequence scenarios. Industry as a whole, however, may not inclined to offer a lot of free time to dedicate to training. Man-hours in drills subtract from productivity. In my opinion, much of industrial management suffers from a lack of imagination in this matter. Safety training and drills are cost overhead. But, what you lack in training hours may be made up for by effective mentoring.
We live in the age of OSHA regulations. Of importance to the process industry is Process Safety Management or PSM. The mission of OSHA is copied and pasted below.
With the Occupational Safety and Health Act of 1970, Congress created the Occupational Safety and Health Administration (OSHA) to assure safe and healthful working conditions for working men and women by setting and enforcing standards and by providing training, outreach, education and assistance.
The Wikipedia link below gives an excellent summary of OSHA regulations relating to the chemical process industry. PSM in 29 CFR §1910.119 titled Process safety management of highly hazardous chemicals, is a regulatory framework covering all aspects of safety management and threshold quantities (Appendix A) of highly hazardous materials. Whether your facility is operating at the PSM scale of operation or not, employers have a duty to assure a safe operating environment for their employees. In my view, PSM regulations frame a safety mindset and diligence that is useful outside of PSM reach. Given that a debilitating injury, fatality, explosion or major fire will bring the unblinking eye of regulators and possible litigation, sensible practices found in 29 CFR §1910.119 that are woven into your chemical safety SOPs are in the direction of goodness. Again, this is my view and should not be construed as legal advice. Your chemical safety plan is your responsibility alone.
Finally, a word to lab managers and supervisors. I cannot point to a ancient stone or a law of nature that commands that leaders be effective instructors and mentors. But I can throw an idea on the table which is that as a senior employee in a supervisory role, you have a moral obligation to your charges to make sure that they practice their art with diligence and in a safe manner. The best way I know of is to train staff thoroughly in lab operations and have high expectations of your staff. Management by wandering around can be very effective in maintaining discipline and keeping tabs on your shop. Besides, you should be walking around and asking questions anyway.
HClO4 – The Meat and Potatoes
There is much to know about the chemistry of perchloric acid digestion beyond it’s renowned acidity and explosive potential. Appreciating the corrosivity and close adherence to standard laboratory techniques are necessary but not always enough. One such circumstance begging for informed action is method development. In researching this topic I was a little surprised to find that many important details are buried in the primary literature. Worse, a few key references are downright difficult to obtain. By important details, I mean whatever information might help define the safe operating window for a given digestion, or, better put, under what circumstances might a digestion procedure transition from hazardous to dangerous.
The major supplier of perchloric acid and perchlorate salts in the USA is GFS Chemicals in Powell, OH. The founder of this company, G. Frederick Smith was, and remains posthumously through his writings, a top authority on the properties of this acid and numerous perchlorate salts as the result of his many decades of research. Laboratory quantities of perchloric acid can be had from GFS and the usual group of research chemical suppliers.
It is easy to find MSDS data and exemplar laboratory safety guides on your browser detailing sensible storage and use policy. Several found in google-space stand out in my opinion as comprehensive perchloric acid safety documents and SOP’s; UC Berkeley; Boston University; MIT; Harvard; British Columbia Code for Mines to name a few. Again, this is my opinion- form your own. If your perchloric acid “policy” is limited to an MSDS document and perhaps a few safety statements found in a procedure, then I would urge someone in your organization to take it upon themselves to dig in a little deeper. Generate SOPs for all aspects of the perchloric acid life cycle in your facility.
There are many accounts of incidents with perchloric acid that should convince even the most refractory skeptic of the potential for a violent release of energy. There is a perchloric acid incident that stands out as an example of the dangers of a chemical ignorance. It happened February 20, 1947, when a large and violent explosion killed 17 people and led the city of Los Angeles to specifically bar the use of perchloric acid (1) through numerous sections of it’s zoning code.
The most common laboratory use of perchloric acid is in the analytical digestion of samples containing a matrix of organic matter, sludge, tissue, biomass or organic chemicals. There are a great many lab procedures to be found by an internet search including Chemical Abstracts (CAS), the AOAC Official Methods of Analysis manual, and ASTM relating to HClO4. Numerous policy and prudent practices documents can be downloaded from well established institutions that outline some very sensible policies regarding the storage, use, and disposal of HClO4. One particularly good source for sample digestion methods across the periodic table is from Inorganic Ventures. Kudos to Dr. Paul Gaines and this company for the quality of their products and their willingness to share their expertise in trace element analysis.
A search of Chemical Abstracts will turn up many research papers giving digestion procedures in the experimental section. However, it is not often made clear how the workers came upon their particular digestion conditions other than from a reference in an earlier procedure. This is because these papers are about the use and not about the chemistry of digestion. Most of the procedure writers will have done their diligence and provide warning about hazards. What may be omitted within papers that use the HClO4 procedure are the boundaries of safe operation and how the reactivity may vary with concentration and temperature.
For greater detail one must look elsewhere and well back into the 20th century. Much useful information on HClO4 and its salts is to be found in papers from the 1930’s thru the 1970’s. Because of their energetic properties, the propellant and explosives folks usually expand on energetic materials including perchlorates, and yes, they go into some great and admirable detail (2). However these sources tend to be thermochemical in nature and perhaps not a lot of immediate help to a bench chemist.
Unlike many other reagents in the laboratory, perchloric acid can have a downside with immediate negative safety consequences. In particular, if one is aiming to develop a digestion procedure for a new type of sample, say, something with a mixed organic/inorganic matrix or certain heteroatoms compounds with nitrogen or sulfur, it behooves the chemist to take a serious interest in rooting out information about the safe operating boundaries of perchloric acid and what kinds of materials may be problematic. A perchloric acid MSDS will inform you of potential safety hazards, hazard classifications, etc., but a well researched and validated procedure can go far towards keeping you out of trouble. I would recommend that at least one person at your organization be more thoroughly educated in the chemistry of perchloric acid digestion, or wet ashing as it is called. Unlike some other strong acids, contact with organics may have immediate explosive consequences. And by explosive I mean violent, deafening, shrapnel-blasting detonations. Hazardous contact can include contact of hot concentrated acid on paper, on sample material, or even contact of perchloric acid vapor on a gloved hand passing through fumes.
There are some particularly comprehensive and broadly informative publications covering perchloric acid chemistry. A more recent work by John Long (3) of GFS is particularly insightful in regard to drawing a line between perchlorate salts and perchloric acid. The 1960 publication Perchlorates: Their properties, manufacture, and uses by J.C. Schumacher (4) contains an informative chapter (Ch 11) on perchloric acid safety. Perhaps the most useful reference is a book available from GFS (5) or Amazon titled Perchloric Acid and Perchlorates, by A.A. Schilt. The 2nd edition in particular contains a great many useful references.
On heating at ambient pressure, aqueous perchloric acid will concentrate by distillation to a constant boiling azeotrope of 72.5 % HClO4 and water. At this composition its number of waters of hydration is slightly greater than two. In the climb from ca 160 °C to a bp of 203 °C at 1 atm, the 72.5 % acid will transition from being “just” a hot super acid to a super acid and a potent oxidizer.
In the gas phase, this acid can decompose via a radical pathway leading to the evolution of Cl2, O2, H2O either abruptly or after an time interval (6). Note that when something quite hot abruptly decomposes to a greater number of moles of gaseous products, there can be plenty of potential for destructive pressure effects.
For the uninitiated, HClO4 is a “super” mineral acid capable of complete dissociation in aqueous concentrations up to about 4 molar (7). The dissociated form in water is H3O+ ClO4-, or oxonium perchlorate. This is normal Brønsted acid behavior in water, but three things set this acid apart from others, even nitric acid: i) due to the extremely weak coordinating ability of the perchlorate anion, the acid proton is extraordinarily mobile and reactive; ii) at room temperature the anhydrous acid will at some point spontaneously explode; and iii) in concentrated aqueous form at elevated temperatures, say > 160 ºC, the acid becomes an increasingly potent oxidizer with temperature.
The perchlorate anion has a central chlorine atom, formally +7, that sits in a tetrahedral array of four O2- anions to make it anionic. On average the negative charge is spread over the surface of the symmetric anion making the negative charge diffuse with the enthalpy of formation unfavorable to close ion pairing. The perchlorate anion is only weakly attracted to a given cation like H3O+ or oligomers and as such, allows the H3O+ (or larger clusters) to reside in a solvent shell unencumbered by tight ion pairing, depending on the nature of the solvent. Perchlorate salts can have very high water solubility and, in the case of magnesium perchlorate, serve as an excellent desiccant. One exception to the high solubility of perchlorates is potassium perchlorate at only 1.5 g per 100 mL H2O at 25 °C.
- “Explosion at O’Connor Electro-Plating Corp.” LA Times, http://framework.latimes.com/2012/02/20/explosion-at-oconnor-electro-plating-corp/#/0 Site viewed on 12/22/16.
- Perchlorates: A review of their thermal decomposition and combustion, with an appendix on perchloric acid, G.S. Pearson; Rocket Propulsion Establishment; October 1968. http://www.dtic.mil/dtic/tr/fulltext/u2/857556.pdf
- Perchlorate Safety: Reconciling Inorganic and Organic Guidelines, J.R. Long; Chemical Health and Safety, 2002, 9(3), 12-18. http://dx.doi.org/10.1016/S1074-9098(02)00294-0
- Perchlorates: Their properties, manufacture, and uses; J.C. Schumacher, editor; Reinhold Publishing, 1960. See Chapter 11, “Safety Precautions in Handling Perchlorates”, E. Levens, 187-222. Download pdf: Do not try to correct the misspelling in the url. https://archive.org/details/pwechloratesthei001740mbp?q=perchloric+acid+and+perchlorates+schilt
- Perchloric Acid and Perchlorates, Second Edition A.A. Schilt and L.C. McBride, 2003 https://www.gfschemicals.com/statics/productdetails/PERCHLORIC_ACID_AND_PERCHLORATES_496.html
- Thermal Decomposition of Perchloric Acid, Gilbert and Jacobs, Combustion and Flame, 1971, 17, 343-353. DOI: 10.1016/S0010-2180(71)80056-1
- Perchloric Acid and Its Salts- Very Powerful Catalysts in Organic Chemistry, Dalpozzo, Bartoli, Sambri, Melchiorre; Chem. Rev, 2010, 110(6), 3501-3551. DOI: 10.1021/cr9003488
