Reuters has reported that the crack spread enjoyed by oil refiners is currently sitting around $37.50. The crack spread is the difference between the price of crude oil and the petroleum products coming from it. This number is an indicator of the profitability of refinery output.
Cracking is a major operation at oil refineries where heavy, long chain hydrocarbons are broken into shorter chain hydrocarbons. Crude oil naturally contains a limited amount of components suitable for modern engines. An important attribute is branching. The goal is to produce the most valuable products from otherwise longer chain, lower value hydrocarbons.
A Scratch in the Surface of Gas Chromatography
The analytical workhorse of the petroleum refinery is the gas chromatograph, or GC. The GC consists of a precisely controlled oven and within it is a coiled, small diameter hollow fiber many meters in length. It is called a capillary GC column. At one end of the column is an injection chamber with a silicone septum that samples are injected through via syringe. This chamber is hot enough to flash evaporate the sample but not so high that it decomposes. For instance, I have usually used a 250 oC injector temperature. A common volume of liquid to be injected is 1 microliter. The sample can be neat or a solution and must be scrupulously free of particles.
Inside the injector is the carrier gas input- helium is often used. A large amount of the vaporized sample is flushed out of the injector leaving only a small quantity of sample to be injected. Connected to the injector is the entrance of the capillary column. The goal is to inject a very narrow plug of sample into the capillary column all at once. After the injection, the detector is activated and the data collection begins. Progress can be followed in real time or not. Once the sample is on the column the GC run must be taken to completion. There is no reset for the column.
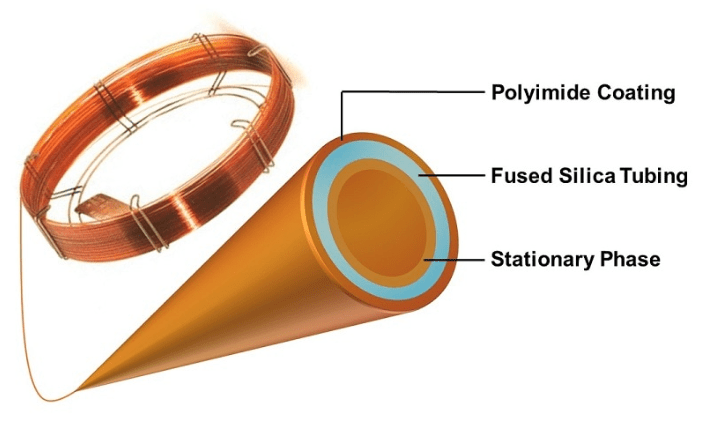
The inside surface of the long capillary column can be just fused silica or it can have a coating. In any case, the components of the sample each have a different affinity for the inner wall of the capillary. As the carrier gas pushes the vaporized sample components along, the components with the least affinity for the inner column surface advance through the column fastest and arrive at the detector earlier. Generally, the higher the molecular weight, the lower the volatility and the longer it takes to exit the column.
At the terminus of the capillary column is the detector. There are a variety of methods used to detect sample and send a signal to the plotter or computer. A particularly useful type of GC system uses a mass spectrometer as a detector. The flow of components enters an ionization chamber and positive ions are generated by electron impact and sent through the mass analyzer and on to the detector. This is occurring continuously as the sample components exit the column. As the components are detected, a regular chromatogram is collected and displayed. The difference with the mass spec detector is that along the timeline, mass spectra are also collected. It is possible to select any given peak in the chromatogram and display the mass spectrum.
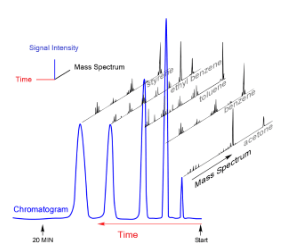
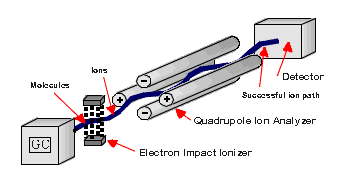
A mass spectrum detector offers the possibility of identifying the individual peaks from the molecular ion mass and the fragmentation pattern. That said, not all mass spectra are easily interpreted. Only cation fragments are visible. Neutral fragments must be inferred.
Back to the Crack
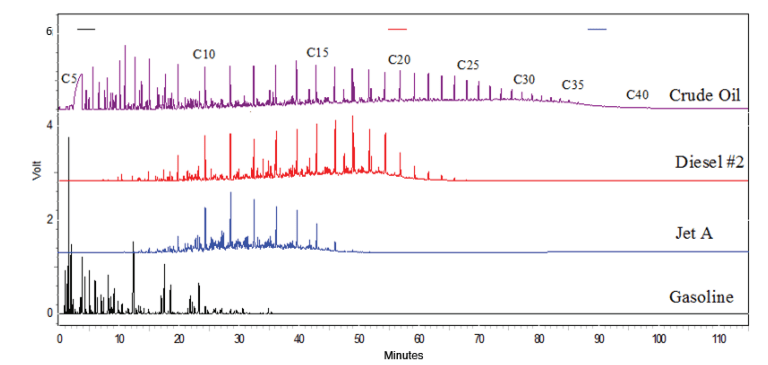
The most valuable refinery products are gasoline, fuel oil (including diesel), and aviation fuel. Within these three areas are subcategories that split into different product lines. These fuel product categories are defined by the number of carbon atoms in the blend of hydrocarbon molecules, saturation, and branching.
Refineries produce blended fuels affording certain properties according to their use. These properties include boiling point and vapor pressure specifications, octane or cetane numbers, viscosity, and pour point specifications. Between distillation, cracking, aromatization and reforming a wide variety of hydrocarbon substances are available from refining for formulation. A refinery is engineered to produce the largest volume of the most valuable hydrocarbons from continuous flow processes at the greatest profit.
Oh, I was just joking about the ACS goons. They don’t bang on your door.